Tosylmethyl isocyanide
| Structural formula | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
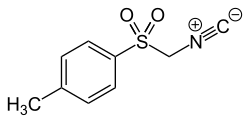
|
||||||||||||||||
| General | ||||||||||||||||
| Surname | Tosylmethyl isocyanide | |||||||||||||||
| other names |
|
|||||||||||||||
| Molecular formula | C 9 H 9 NO 2 S | |||||||||||||||
| External identifiers / databases | ||||||||||||||||
|
||||||||||||||||
| properties | ||||||||||||||||
| Molar mass | 195.24 g mol −1 | |||||||||||||||
| Physical state |
firmly |
|||||||||||||||
| Melting point |
111–113 ° C (decomposes) |
|||||||||||||||
| solubility |
almost insoluble in water |
|||||||||||||||
| safety instructions | ||||||||||||||||
|
||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||
Tosyl methyl isocyanide (TosMIC) is a chemical compound . It is also known as the Van Leusen reagent because it can be used, for example, in the Van Leusen reaction for the synthesis of nitriles. In addition, TosMIC can be used to synthesize oxazoles and imidazoles .
properties
TosMIC has on carbon - atom between the isonitrile, and the sulfonyl group acide hydrogen -atoms. TosMIC receives a nucleophilic center there through deprotonation . The isocyanide carbon is an electrophilic center.
synthesis
TosMIC is obtained in a two-step reaction. First, sodium p -toluenesulfinate , formaldehyde and formamide react to form N - (tosylmethyl) formamide , which is then dehydrated with phosphorus oxychloride to form TosMIC.
Further use
TosMIC is also suitable for the synthesis of pyrroles , indoles , 1,2,4-triazoles and thiazoles .
Individual evidence
- ↑ a b Tosylmethyl isocyanide data sheet (PDF) from Fisher Scientific , accessed on February 13, 2014.
- ↑ a b Data sheet p-Toluenesulfonylmethyl isocyanide, 98% from Sigma-Aldrich , accessed on July 14, 2013 ( PDF ).
- ↑ Daan Van Leusen, Albert M. Van Leusen: Synthetic Uses of Tosylmethyl Isocyanide (TosMIC) . In: Organic Reactions . tape 57 , no. 3 , 2004, doi : 10.1002 / 0471264180.or057.03 .
- ^ Jie Jack Li: Name reactions: A collection of detailed mechanisms and synthetic applications , 5th edition, Springer, Heidelberg, 2014, pp. 613–614, ISBN 978-3-319-03979-4 .
- ↑ TosMIC (PDF; 56 kB).
- ↑ Otto H. Oldenziel, Daan Van Leusen, Albert M. Van Leusen: Chemistry of sulfonylmethyl isocyanides. 13. A general one-step synthesis of nitriles from ketones using tosylmethyl isocyanide. Introduction of a one-carbon unit. In: The Journal of Organic Chemistry . Vol. 42, No. 19, 1977, pp. 3114-3118 doi : 10.1021 / jo00439a002 .
- ↑ BE Hoogenboom, OH Oldenziel, AM van Leusen: p-tolylsulfonylmethyl isocyanide in: Organic Syntheses . 57, 1977, p. 102, doi : 10.15227 / orgsyn.057.0102 ; Coll. Vol. 6, 1988, p. 987 ( PDF ).

