FeLV infection
An infection with the feline leukemia virus (FeLV) can cause serious illness in cats. The virus from the Retroviridae family , which is mainly transmitted from cat to cat through the saliva, causes severe disease symptoms in some of the infected animals, which can be very variable. In addition to lymphomas , i.e. malignant tumors of the lymphatic tissue , the most common symptoms are a weakened immune system and anemia .
Not all infected animals develop clinical symptoms. Cats with sufficient immune competence manage to overcome the infection at an early stage. In the other animals, after infection, the virus first gets through the oral and nasal mucous membranes into the lymphatic tissue of the nasopharynx, from where it spreads through the bloodstream to the bone marrow , where it infects the blood-forming stem and progenitor cells and finally with the blood cells it is distributed all over the body. In the body of this as " progressively infected.""Cats named, there is a constant multiplication of the virus. Large amounts of virus particles can be detected in the blood, which are excreted in the body fluids, especially in the saliva, so that these cats represent a source of infection for other animals.
As a retrovirus, the feline leukemia virus integrates its genetic material into the infected host cells, which thus contain all the information necessary for the virus to multiply in the form of a so-called provirus . In this way, the virus can no longer be eliminated from an infected cat's body. There is therefore no causal treatment of FeLV infection, so that palliative therapy can only alleviate the symptoms.
Vaccines against the feline leukemia virus are available; they reliably protect cats from the development of a progressive infection and thus from the development of symptoms, but they cannot completely prevent infection with the virus. Vaccination is only recommended for those cats that have potential contact with other cats with unknown FeLV status.
terminology
Infection with the feline leukemia virus is often in cats with tumors of the white blood cells associated why associated with FeLV infection diseases often referred to as feline leukemia or feline leukemia are called. However, since the FelV infection can be accompanied by numerous other and very variable symptoms, this term should not be used synonymously for the FeLV infection.
In German-speaking veterinary medicine, the term leukosis is often used for systemic neoplastic proliferation of white blood cells or their precursors . Solid tumors of the hemolymphatic tissue, such as the lymph nodes , the thymus or the spleen , are also often included. If tumor cells are present in the blood, one speaks of a leukemic leukosis ; if, on the other hand, no tumor cells can be detected in the bloodstream, one speaks of aleukemic leukosis .
According to this concept uses is often called the FeLV infection in German-speaking feline leukemia and the causative virus misdiagnosed as feline leukemia virus called.
Above all in English-speaking countries, the term leukemia (leukemia), which is used in human medicine, is used for malignant diseases of the blood-forming or lymphatic system, however, also in veterinary medicine . Just as with the term leukosis, this term also includes solid tumors of the lymphatic tissue, which explains the name of the virus as feline leukemia virus , although William Jarrett discovered it in cats with lymphoma and not leukemia were sick.
history
The FeLV virus was first isolated in 1964 by William Jarrett, a veterinary pathologist at the University of Glasgow . A Glasgow veterinarian made him aware of the high prevalence of lymphoma among the cats he treated. Jarrett realized that certain groups of cats that lived in close contact with one another had an increase in these tumors. In a group of cats that lived together in one household, he found leukemia in eight animals. In another household with several cats, the previously healthy animals developed more tumors after a new breed tomcat was brought into the group. The observation that healthy kittens also became ill after coming into contact with sick puppies led him to conclude that there must be a tumor-inducing agent that can be horizontally transmitted from animal to animal.
The concept of virus-induced tumorigenesis had been known since the description of the Rous sarcoma virus in chickens in 1911 by Francis Peyton Rous , who was awarded the Nobel Prize in Medicine in 1966 for his discovery . In the 1930s, retrovirus- induced leukemias in mice and the Shop's fibromavirus ( Leporipoxvirus fibromatosis ), which belongs to the family of Poxviridae and which induces mesenchymal tumors in rabbits , were described
Jarrett and his colleagues succeeded in demonstrating that lymphomas could be transmitted from one animal to another, and in isolating viruses from tumors of the spleen, thymus and mesenteric lymph nodes of a cat with lymphosarcoma and growing them in a cell culture .
After the murine leukemia virus , the feline leukemia virus was the second retrovirus described that can induce tumors in mammals. After a number of animal retroviruses had been discovered that cause malignant diseases (including the bovine leukemia virus BLV, the avian leukosis viruses ALV, mouse mammary tumor virus MMTV, and others), many medical professionals were of the opinion that it was only a matter of time until appropriate human oncogenic retroviruses were found. However, when this did not happen for decades or when there were repeated false reports and false alarms, the research community grew skeptical. It was not until the late 1970s that the working group led by the American virologist Robert Gallo succeeded in discovering the first two human retroviruses, HTLV-1 and HTLV-2 . When Gallo dealt with the "epidemic" AIDS , which appeared in the early 1980s , he initially thought a FeLV-like retrovirus could be the trigger, as he remembered the immunosuppression that sometimes occurs with FeLV infection. Ultimately, however, a new lentivirus - HIV - was identified as the agent.
Feline Leukemia Virus
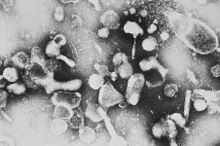
The feline leukemia virus belongs to the retrovirus family . As a gammaretrovirus , it contains a single-stranded RNA which, when the virus replicates after infection of a host cell, is first transcribed into double-stranded DNA by the virus’s own enzyme, reverse transcriptase , and then integrated into the genome of the host cell. The integrated virus DNA is called provirus and is the template for the synthesis of new virus particles. The virus genome and thus the complete information for virus replication is anchored in the host cell via this mechanism; the host organism can no longer eliminate the virus from the body.
The virus replication cycle includes numerous possibilities for genetic variability to develop . The process of translating RNA into DNA is relatively error-prone, since the enzyme reverse transcriptase has no control function. This regularly results in mutations in the virus genome, which can lead to new genetic variants. In addition, there is regular recombination with gene segments of the host cell genome.
The virus genome integrated into the cell genome as a provirus is passed on to daughter cells during cell division. In the course of evolution, complete endogenous FeLV proviruses have been established at various loci in the domestic cat genome , which are passed on to subsequent generations via the germline. Transcription products of these endogenous viruses could be detected in various organs of the cat, so that if the cell is infected with FeLV-A, a recombination between the exogenous FeLV-A and the endogenous FeLV transcription products can occur.
Due to the high rate of mutations and recombinations in virus replication, the FeLV represents a group of genetically very closely related viruses that are characterized by a high genetic variability . A distinction is made between the four subtypes FeLV-A, FeLV-B, FeLV-C and FeLV-T, which differ in the sequence of the env gene, the receptors used in the infection of host cells and their cell tropism .
Of the four subtypes, FeLV-A is the transmissible and infectious form of the feline leukemia virus and the only infectious subtype per se . It can be detected in all naturally infected cats, with numerous different genetic variants having been isolated so far.
The FeLV-B subtype arises de novo in cats infected with FeLV-A through recombination between FeLV-A and sequences of the endogenous FeLV- env gene of the host cell. It can only be transmitted from cat to cat together with the FeLV-A subtype. Cats infected with the FeLV-B subtype are more likely to develop lymphomas than animals infected with FeLV-A alone. The prognosis is much worse.
The FeLV-C subtype arises de novo in FeLV-A infected cats through a mutation. Infections with the subtype FeLV-C cause severe, fatal non-regenerative anemia . However, it rarely occurs and cannot be transmitted from cat to cat. Infections with the fourth subtype FeLV-T are associated with a severe weakening of the immune system and physical decline.
Distribution and Epidemiology
Host spectrum
FeLV infection is common worldwide. The Feline Leukemia Virus has a relatively strict host specificity for species from the family of cats (Felidae) . In addition to the domestic cat ( Felis catus ) , FeLV infections have also been reported in wild cats ( Felis silvestris ) , the Eurasian lynx ( Lynx lynx ) , the Iberian lynx ( Lynx pardinus ) , the puma ( Puma concolor ) and the Chilean wildcat ( Leopardus guigna) ) proven. It is not infectious for non-felids, so there is no risk of infection for humans.
FeLV infections are rare in non-domesticated species of the Felidae family. Most of the infections in wild animals described so far have been detected in animals in captivity and could be traced back to direct contact with FeLV-infected domestic cats. In almost all post-controlled cases, the infected animals were able to completely eliminate the virus, which is why the virus was not transmitted to other conspecifics here.
Since no endogenous FeLV viruses have been found in any other cat species than domestic cats , it was postulated that FeLV can infect various species of the cat family, but is only pathogenic for domestic cats. This hypothesis had to be revised after a FeLV outbreak in the Florida panther ( Puma concolor coryiin ) population in 2001-2006 and the detection of FeLV-infected Iberian lynx in Spain in 2004-2007, after both cases several animals were known to have died from the infection. Since both outbreaks affected populations of a species threatened with extinction, and FeLV viraemia was detected in several clinically diseased and dead animals, FeLV infection is now also viewed as a danger to the conservation of threatened species, especially if only now small residual populations live in limited geographical areas and with close contact between the animals.
Prevalence
In Europe, the United States, and Canada, around 1% -10% of domestic cats are infected with the virus. The prevalence is mainly influenced by the density of a cat population and the specific housing conditions of the cats, so that there are large geographical and local differences. In most countries, less than 1% of cats kept individually are infected with the virus. In households in which several cats are kept in close contact with each other and in which no special precautionary measures are taken against the transmission of the infection, as well as in groups of feral domestic cats, the prevalence can exceed 20%. In cats that were tested during an illness, infection rates of up to 30% could be detected, which indicates the special importance of the virus as a pathogen.
Up until the 1990s, around a third of all tumor-associated deaths in cats could be traced back to FeLV infection. Since numerous animals also died of FeLV-associated anemia and secondary infections caused by the weakened immune system, the FeLV infection was responsible for the majority of disease-related deaths in cats during this time.
In the last few decades the prevalence and thus the importance of the feline leukemia virus as a pathogen has decreased significantly. This is due to the availability of reliable diagnostic tests and vaccines, extensive testing and elimination programs, and a better understanding of the pathogenesis and course of infection.
transmission
The virus can be transmitted both horizontally and vertically.
Viremic cats excrete large amounts of the virus in saliva, nasal secretions, tears, feces, urine, and breast milk. The infection usually occurs through direct contact with infected animals, especially through the saliva. The indirect transmission through objects contaminated with virus-containing saliva or other body fluids, such as feeding or drinking bowls, is of little importance because the virus is deactivated outside the cat at room temperature within a few minutes. But it can occur under poor hygienic conditions, if z. B. eat several cats from one bowl at the same time. The most important mode of transmission is friendly contact between cats, such as licking each other to groom. But the infection can also be transmitted through bites or even just bite attempts in which saliva is transmitted, for example during rank fights or during mating.
The virus is transmitted vertically from viremic pregnant cats, i.e. the fetuses can be infected through the placenta (transplacental). However, this usually leads to their death and / or miscarriages . Transplacentally infected puppies already show viraemia at birth, worry and die after a short time ( fading kitten syndrome ).
With increasing age, cats become increasingly resistant to FeLV infection, so that kittens and kittens are particularly at risk of infection. Free range cats are particularly at risk of FeLV infection as they can have contact with animals with unknown FeLV status. This is especially true for uncastrated animals, as they are more often involved in territorial fights and the infection can also be transmitted through close contact during mating. Further risk factors for an infection are keeping cats in larger groups with changing casts, a high local cat density and poor hygienic conditions.
Course of infection
Feline leukemia virus infection has a chronic course and is characterized by a long asymptomatic phase in which the affected cats show no clinical signs of disease.
After the virus is absorbed through the oral or nasal mucosa, it first multiplies in the local lymphatic tissues of the nasopharynx . The first viraemia occurs just a few days after infection , so that virus particles bound to lymphocytes and monocytes can be detected in the peripheral blood. Within 7 to 14 days, other lymphatic organs are infected via the bloodstream, mainly the thymus , other regional lymph nodes and the Peyer's plates of the small intestine. 14 to 21 days after the first virus contact, infected cells can also be detected in the bone marrow , which produce large quantities of virus particles that are washed out into the bloodstream and thus cause a second, significantly stronger viraemia.
In principle, all hematopoietic cells can be infected by the FeLV. While initially mainly lymphocytes and monocytes are infected, in the later course of the infection the neutrophils and platelets show the strongest virus replication.
Gradient forms
Infection with the FeL virus can take various forms. While it used to be assumed that cats that had survived FeLV infection without developing persistent viraemia were immune to FeLV infection, it is now known from more sensitive diagnostic methods that most cats who have FeLV infection After going through infection, remain provirus positive. So far, however, it is unclear whether this provirus-positive status is of clinical relevance and what epidemiological significance these animals have. The virus cannot be detected in the blood of these animals by a test for viral protein, nor do they excrete it. However, since the virus is integrated into the genome of host cells in the form of the provirus, it cannot be eliminated from the body. After reactivation of virus replication, however, a renewed viraemia and the excretion of infectious viruses can in principle occur.
Based on the knowledge that there are numerous animals that react negatively in the test to virus antigens in the blood, but are also provirus-positive, a new classification of the forms of the FeLV infection was made. According to this classification, the infections are divided into an abortive, a progressive, a regressive and an atypical or focal infection.
The course of the infection is mainly determined by the immune response of the infected animal. Cats with an abortive or regressive course of infection have high levels of circulating FeLV-specific cytotoxic T lymphocytes (CTL) in their blood even before virus-neutralizing antibodies appear. Progressively infected cats with persistent viraemia, however, only react to the infection with a reduced specific cell-mediated and humoral immune response.
The age at the time of infection is considered to be one of the most important characteristics of the infected animal that determine the course of the infection. After an infection with the feline leukemia virus, newborn kittens develop thymus atrophy with severe immunodeficiency, worry and die after a short time. Resistance to the infection develops progressively with age. Older cats usually show an abortive or regressive course of infection. If they do develop a progressive infection, usually only mild symptoms develop and the phase until the onset of the first symptoms is significantly longer.
Abortive infection
In abortive infection, the virus multiplies in the local lymphatic tissues of the nasopharynx after infection. Cats with sufficient immune competence are able to develop an effective humoral and cell-mediated immune response so that the virus does not spread further through the bloodstream in the body. A high level of neutralizing antibodies directed against the virus is present in the blood of these animals , while FeLV protein, viral RNA or proviral DNA cannot be detected at any time.
Under natural infection conditions, FeLV infection is abortive in around 20% to 30% of cats. In addition to sufficient immune competence, the prerequisite is probably only a low infection pressure from low doses of FeLV to which the animals are exposed. According to the previous classification, cats in which no virus antigen could be detected in the blood after infection were called regressor cats. With the help of the very sensitive polymerase chain reaction , proviral DNA could actually be detected in tissue samples from numerous cats that had previously been classified as regressor cats, which shows that only some of the infected cats are actually able to completely remove the FeLV to eliminate from the body.
The abortive infection can only be detected by detecting antibodies directed against FeLV in the blood ( seroconversion ). Since the virus is completely eliminated from the body, an abortive FeLV infection has no clinical consequences in later life. No virus is excreted, so once the animals have overcome the infection, they are not a source of infection for other cats.
Cats who have had an abortive FeLV infection will remain immune to re-infection with the virus for life.
Progressive infection
In a progressive infection, the virus goes through the full course of infection from the local lymph nodes to the infection of the bone marrow cells. The host organism does not succeed in developing a sufficient immune response to the infection, so that there is a continuous virus replication with constant re-infection of further body cells. Progressively infected cats therefore have persistent viraemia, and large amounts of virus protein can be detected in their blood at any point in time after the infection of the bone marrow. The animals permanently excrete infectious virus particles through their saliva, but also through tears, faeces, urine and breast milk, so that they represent a source of infection for other cats.
About 30% to 40% of FeLV infected cats develop progressive infection with persistent viraemia. Several factors determine whether a cat develops progressive infection. Infection of young cats up to 16 weeks of age leads to lifelong viremia in the vast majority of cases. If the infection pressure is high or the immune response is insufficient, older animals can also become permanently viremic.
Most progressively infected cats will develop FeLV-associated clinical disease within months to years of infection. Cats with a progressive infection therefore have a poor prognosis with a significantly reduced life expectancy.
Regressive infection
A regressive course of infection develops in 30% -40% of the cats infected with FeLV. After the initial infection and an initial replication in the local lymphatic tissues, the virus is initially distributed throughout the body by lymphocytes and monocytes via the bloodstream (first viraemia). At this stage of the infection, virus antigen can be detected in the cats' blood and the cats excrete virus mainly through their saliva. In the further course of the infection, however, the animals succeed in preventing virus replication and thus the infection of other body cells through an adequate immune response. The infection can be contained before the infection of the bone marrow, or in rarer cases during the second viraemia. This can be done within weeks or, in exceptional cases, months after the infection.
The molecular basis of the regressive infection is the integration of a copy of the viral genome (provirus) into the chromosomal DNA of the host cells. As a result, the virus cannot be completely eliminated from the body; the genetic information for virus replication remains in the body's cells. With each division of a cell carrying the provirus, however, the proviral DNA is multiplied and the genetic information is passed on to the daughter cells that are formed, so that all daughter cells resulting from the infected progenitor cells contain proviral FeLV DNA.
Although the proviral DNA is present in the host cell, no virus is produced by the cells, which is why no FeLV antigen can be detected in the blood of the affected animals. With the help of a PCR , however, the proviral DNA can be detected in host cells. The animals do not excrete a virus and are therefore not a source of infection for other cats.
Since the complete genetic information for the formation of virus particles is available in the host animals, a regressive infection can be reactivated in the event of a weakened immune system, because the immune response becomes too weak to further suppress virus replication. The can z. B. by chronic stress, the administration of glucocorticoids or other immunosuppressive drugs or an infection with the feline immunodeficiency virus . The reactivation can lead to renewed viraemia and thus to the excretion of infectious viruses. According to the earlier classification, the regressive infection was therefore called latent infection. However, reactivation of a regressive FeLV infection is likely to be extremely rare under natural conditions.
The clinical relevance of regressive infection is not yet fully understood. What is certain is that regressively infected cats have a significantly lower risk of developing FeLV-associated diseases compared with progressively infected animals. Compared to cats that have never had contact with FeLV, however, they have a slightly higher risk of developing lymphoma in the course of life. In virus replication, provirus is integrated into the cell genome of every infected cell, so that tumor induction can occur. However, since significantly fewer body cells are affected in a regressive infection than in a progressively infected cat with persistent virus replication, the risk of malignant degeneration of host cells is lower. Lymphomas usually only develop in regressively infected animals at a significantly older age and there is an increased incidence of B-cell lymphomas.
Cats with regressive infection are immune to re-infection with the FeLV. They usually have high levels of virus-neutralizing antibodies for years, even if they never come into contact with the virus again.
Focal or atypical infection
The focal or atypical infection is characterized by a persistent virus replication that is locally limited to certain tissues. For example, individual mammary glands, the urinary bladder or the mucous membranes of the eyes can be affected. Since the virus multiplies only in certain tissues, there are usually only small amounts of virus in the blood, and detection of virus protein is usually only weak. In some cases the virus replication only takes place intermittently, so that the detection of the affected animal is only positive at certain times. The virus is excreted locally, depending on the tissue affected, often intermittently and usually in lower amounts than in acutely viremic or progressively infected cats.
After experimental infection, up to 10% of cats show an atypical course of infection, but it is assumed that this course of infection occurs less frequently under natural conditions.
Since the affected animals only have local or intermittent virus replication and thus also excretion, they are often difficult to identify diagnostically, but represent a potential source of infection for other cats due to the intermittent virus excretion.
Clinical picture and symptoms
The naming of the virus as feline leukemia virus came after the observation that cats infected with the virus often develop white blood cell tumors, i.e. leukemia . However, the clinical effects of FeLV infection are not limited to the formation of tumors of the hematopoietic cells, nor are these the most common diseases associated with FeLV infection. The possible clinical consequences of a FeLV infection are extremely varied, especially since the infection often causes an immune deficiency that can lead to non-specific secondary infections. The Feline Leukemia Virus is responsible for more different clinical symptoms and syndromes in cats than any other known single pathogen. The most common clinical consequences of FeLV infection are immunodeficiency, anemia, and lymphoma.
The question of which symptoms occur in the course of the infection is determined on the one hand by the properties of the virus strain causing the infection and on the other hand by the properties of the infected animal. Infections with viruses of the FeLV-B subtype are associated with the development of tumors, while infections with the FeLV-C subtype trigger a non-regenerative anemia.
Clinical symptoms occur especially in progressively infected cats, as new body cells are constantly infected by the persistent virus replication. In comparison, regressively infected cats have a significantly lower risk of developing clinical symptoms, which usually only appear after a significantly longer latency period.
Clinically, a distinction is made between neoplastic diseases, which are associated with the formation of various tumorous changes, and non-neoplastic diseases. The non-neoplastic FeLV-associated diseases and syndromes include pathological changes in blood cells, immunodeficiency, immune-mediated diseases and other syndromes and symptom complexes, such as neuropathies or reproductive disorders .
FeLV-associated neoplasms
The neoplastic form is characterized by the development of tumors. FeLV infection mainly causes lymphomas and leukemia, and less often other tumors of the haematopoietic system. Other malignant tumors such as B. neuroblastomas or osteochondromas .
Oncogenesis by FeLV
After infection of a host cell with FeLV, a DNA copy of the virus genome is integrated into the genome of the host cell. This insertion takes place randomly at different locations in the host genome. Depending on where in the genome the virus DNA is inserted, changes in the expression of various genes in the host cell can occur. If a proto-oncogene is affected, this can lead to a malignant degeneration of the host cell, which passes on the genetic information including the FeLV genome to the daughter cells that are formed during cell division.
Proto-oncogenes are genes whose transcription products play an important role in regulating cell growth, cell division and cell differentiation. They code, for example, for growth factors , growth factor receptors or protein kinases , which, when expressed in an uncontrolled manner, promote cell proliferation . Their expression is subject to finely tuned genetic control mechanisms. Do these mechanisms go e.g. B. lost through a mutation, there is an uncontrolled expression of the proto-oncogene, which results in uncontrolled cell growth or changes in cell differentiation.
In the replication cycle of FeLV, the virus gnome is integrated into the genome of the host cell. If this happens in the immediate vicinity of a cellular proto-oncogene, the viral promoter takes over the regulation of the expression of this gene and stimulates it to constant expression, which can lead to uncontrolled cell proliferation and thus the development of a tumor.
Another mechanism of tumor induction by FeLV is the loss of a tumor suppressor gene through the insertion of the virus genome. This eliminates a genetic control mechanism that inhibits gene expression, which also leads to uncontrolled expression of the previously controlled oncogene. This mechanism occurs less often, however, since two alleles of a gene are present in the cell and both suppressor genes have to fail before their function is lost.
A recombination between the genome of FeLV and the cellular DNA of the host cell can lead to the formation of recombinant viruses. Cellular oncogenes can also be integrated into the virus genome, which are then integrated into their cell genome when other host cells are infected. Since the location of the insertion is determined by chance, the newly inserted oncogene is no longer subject to the physiological control of the associated transcription unit, which leads to uncontrolled transcription of the gene product, which can result in a malignant transformation of the corresponding host cell.
Recombinant viruses can often be isolated from feline lymphoma cells which have arisen from a recombination between the FeLV virus genome and the Myc oncogene of the host cell. These recombinant viruses are accordingly referred to as FeLV / myc . myc codes for various transcription factors , so it plays an important role in controlling gene expression . 10% to 15% of FeLV-associated lymphomas contain myc-transducing virus, in thymic lymphomas it is even almost 30%.
Another recombinant virus often isolated from feline lymphomas is FeLV / tcr , which results from recombination with the gene for the cellular T cell receptor . T cell receptors are anchored on the surface of lymphoid cells and play an important role in recognizing antigens and regulating the immune response. Their activation leads to changes in cell differentiation and gene expression. After the transfer of the tcr gene by the recombinant FeLV / tcr into the genome of a host cell, the T cell receptor can be overexpressed with increased cell proliferation due to autocrine stimulation and thus malignant degeneration of the cell.
In persistent viremic cats, further body cells are constantly infected, each of which is associated with an integration of the virus genome into the genome of the host cell. The likelihood of malignant degeneration due to an insertion mutation or due to a recombination between the virus genome and cellular oncogenes increases with the number of newly infected cells. In the tumor cells from most FeLV-associated lymphomas, there are several different genetic lesions induced by the virus, which usually affect more than one gene locus. So far, in the genome of lymphoma cells from FeLV-infected cats, 12 sites at 6 different gene loci at which the virus DNA was integrated could be identified.
The feline sarcoma virus (FeSV) is a special case of recombination between FeLV and cellular gene segments. This occurs de novo in FeLV-infected cats through recombination with cellular oncogenes. Since a large part of the virus genome is replaced by cellular oncogenes, the FeSV is replication-defective and relies on FeLV-A as a helper virus for reproduction .
Lymphoma
Lymphoma is the most common malignant tumor in domestic cats worldwide. By the 1980s, up to 80% of lymphomas diagnosed in cats were directly attributed to FeLV infection. Since then, the proportion of FeLV-associated lymphomas in the total number of diagnosed lymphomas has decreased significantly. Today, only about 13-15% of lymphomas diagnosed in cats are related to FeLV infection, which is due to the greatly reduced prevalence of FeLV infection in the cat population. Interestingly, however, this development is not associated with a decrease in the overall incidence of feline lymphoma.
60% to 80% of FeLV-infected cats that develop lymphoma are progressively infected and therefore have persistent viraemia. In young cats under one year of age diagnosed with lymphoma, even 90% show FeLV viraemia, whereas in older cats over 7 years of age it is only 50%.
Cats who are progressively infected with FeLV are 60 times more likely to develop lymphoma or leukemia than cats who have never had contact with the virus. The most common are T-cell lymphomas . Mediastinal or thymic lymphomas often occur in connection with FeLV infection, especially in young cats under 3 years of age . Alimentary or gastrointestinal lymphomas, on the other hand, are mainly observed in older cats and usually cannot be attributed to FeLV infection. Even spinal shapes are diagnosed regularly.
The general signs of lymphoma are nonspecific and include listlessness (lethargy), loss of appetite (anorexia), and weight loss. The other symptoms depend on the affected organ system. In alimentary lymphomas, the small intestine, appendix and colon can be affected, the animals mainly show vomiting and diarrhea. In the multicenter form, there is a generalized disease of the lymph nodes (lymphoadenopathy), lymphosarcomas of the kidneys and enlargement of the spleen and / or liver also occur. In the thymus form, swallowing disorders (dysphagia) and shortness of breath (dyspnea) due to compression of the esophagus and windpipe can occur due to the tumorous enlargement of the organ. Often neoplastic cells can be detected in the pleural fluid.
In the lymphoid-leukemic form, the bone marrow is primarily affected; degenerate lymphocytes circulate in the blood (leukemia). The main symptoms are weakness, anorexia, jaundice, fever, anemia, and pale mucous membranes. In addition, disease of the lymph nodes, enlarged spleen (splenomegaly) and enlarged liver (hepatomegaly) can occur.
Leukemias
Leukemia is a malignant disease of the blood-forming or lymphatic system. In principle, all hematopoietic cells are susceptible to FeLV infection, so that both lymphatic and myeloid ( granulocytic , erythroic and megakaryocytic ) leukemias can occur in association with FeLV infection. If FeLV infection occurs at the stem cell level , more than one row of cells can be affected.
FeLV infection can be detected in approximately 50% of cats diagnosed with leukemia.
FeSV-induced tumors
Fibrosarcomas induced by the feline sarcoma virus (FeSV) represent a special case of FeLV-associated tumors . FeSV arises de novo in cats infected with FeLV-A through recombination of FeLV-A with protooncogenes of the host cell. It has a replication defect, which is why it depends on the presence of FeLV-A as a helper virus in order to be able to infect other body cells. This means that FeSV-associated fibrosarcomas can only develop if there is a simultaneous FeLV-A infection with persistent viraemia.
Because of the replication defect, the Feline Sarcoma Virus cannot be transmitted horizontally from cat to cat. FeSV carrier animals are therefore not a source of infection for other cats.
Since the FeSV integrates protooncogenes into the genome of the host cell in every replication cycle, it induces uncontrolled proliferation in the infected cells and thus has acute tumor-inducing properties. Affected animals develop after a short incubation period polyclonal fibrosarcoma low degree of differentiation , which occur at the same time or at short intervals in various parts of the body and organs. They occur primarily as multiple skin and subcutaneous nodules in young cats and are prone to rapid and invasive growth and metastasis to the lungs and other organs.
FeLV-Associated Non-Neoplastic Diseases and Symptoms
Changes in blood cells

Haematological changes that have been described in connection with FeLV infection are primarily based on a disruption of the formation of blood cells in the bone marrow ( myelosuppression ). They include non-regenerative and regenerative anemias, persistent, transient or cyclic neutropenia , pathological changes in platelets ( thrombocytopenias and abnormalities in platelet function), aplastic anemias ( pancytopenia ), and syndromes similar to panleukopenia .
The pathological mechanisms by which FeLV causes bone marrow suppression are not yet fully understood. On the one hand, the incorporation of the FeLV provirus into the cell's own gene sequences causes the disintegration and thus inactivation of genes. On the other hand, the expression of neighboring gene sequences can also be changed by the viral DNA. Myelomonocyte progenitor cells and stromal fibroblasts , which play an important role in the microenvironment of the bone marrow, can be disturbed in their function after infection with FeLV and the associated provirus DNA, which leads to disturbances in homeostasis in the bone marrow. In addition, FeLV infection can induce the expression of antigens on the cell surface of progenitor cells, which leads to immune-mediated destruction of the cells.
Anemia occurs in more than 50% of FeLV infected and diseased cats. Only about 10% of FeLV-associated anemias are regenerative, the vast majority, however, are non-regenerative. The most important pathomechanism in the development of FeLV-associated anemia is the direct infection of the hematopoietic stem cells and the stromal cells, which are responsible for the environment surrounding the hematopoietic cells. In addition, other factors can cause non-regenerative anemia in FeLV-infected cats. The chronic infections caused by the infection can lead to high cytokine levels that ultimately lead to anemia. In FeLV-associated leukemias, the proliferating tumor cells in bone marrow can displace the other precursor cells, and thus the formation of red blood cells suppress ( English crowding out ).
In particular, the infection with the FeLV-C subtype causes massive bone marrow depression through a disruption of cell division, which leads to severe aregenerative anemia, the so-called pure red cell aplasia . Macrocytosis of the erythrocytes with a simultaneous absence of reticulocytes can be observed in the affected animals . In an advanced stage of FeLV-C infection, the animals often suffer from aregenerative pancytopenia.
Many progressively FeLV-infected cats have a reduced number of blood platelets ( thrombocytopenia ) which, depending on the extent, leads to an increased tendency to bleed. Thrombocytopenia can be caused by reduced platelet production due to infection of the platelet precursor cells ( megakaryocytes ) or a leukemic infiltration of the bone marrow. On the other hand, immune-mediated destruction with shortened survival time of the platelets can occur, which is often associated with immune-mediated hemolytic anemia. In addition to a reduced platelet count, a disorder of the platelet function can also occur.
In addition to changes in the red blood count and platelets, the FeLV infection can also lead to disorders of the white blood count. A decrease in neutrophil granulocytes is often diagnosed in FeLV-infected animals . In some cases hypoplasia of all granulocyte stages can be observed, which is probably due to an infection of the neutrophil progenitor cells. Since in some cases the neutrophil count recovers after the administration of glucocorticoids, an immune-mediated mechanism in the development of neutropenia is also discussed.
In many persistently viraemic cats, the neutrophils also show reduced chemotaxis and reduced phagocytic function , so that in addition to the pure reduction in the number of cells there is also a functional disorder.
Neutropenia can occur in isolation or in association with a decreased number of lymphocytes . The FeLV can replicate directly in the lymphocytes. Usually both the T helper cell and the cytotoxic T cells are affected by lymphopenia; however, an increased loss of CD4 -positive T helper cells is also observed, so that the CD4 + / CD8 + ratio is reversed.
The feline panleukopenia-like syndrome (FPLS) , which is also under the name FeLV-associated Enetritis or Myeloblastopenie is known, by a severe leukopenia (<3000 cells / mm), a enteritis in and destruction of the epithelium of the intestinal crypts. The symptoms are similar to those by the by the Feline panleukopenia virus (FPV) caused feline panleukopenia and express themselves in bloody diarrhea, vomiting, ulcers of oral mucosa, gum inflammation, loss of appetite and weight loss. It is unclear whether this syndrome is caused directly by the FeLV infection or whether it is a co-infection with the FPV.
Weakening of the immune system and secondary infections
Regardless of whether the animals show clinical symptoms of FeLV infection, every cat with persistent FeLV viraemia is immunocompromised and suffers from a delayed and reduced primary as well as secondary immune response.
The mechanisms behind FeLV-associated immunodeficiency are very complex and varied. The frequently occurring changes in the white blood count such as neutropenia, lymphopenia, neutrophil dysfunction, the loss of CD4-positive T-cells and / or CD8-positive lymphocytes lead to a weakening of both the cellular and the humoral immune response . This results in a reduced ability of the infected animals to react immunologically adequately to infections and other noxious substances .
Many FeLV-infected cats also show changes in cytokine production . The various cytokines play an important role in coordinating the immunological response. Changes in their interaction therefore lead to disorders of the immunological defense, which can result in both a reduction and an excessive immunological reaction. While some cats show reduced interleukin-2 and interleukin-4 levels, interleukin-1 production is not affected by FeLV infection. Compared to healthy cats, the T cells of infected cats produce significantly less B cell-stimulating factor (interleukin-6) . The level of interferon-γ can be decreased or increased. An increased level of tumor necrosis factor-α (TNF-α) is also often detected in the serum of infected cats . These changes in cytokine concentrations can contribute to a dysregulation of the immune system.
The weakening of the immune system is the most common and most important clinical consequence of FeLV infection. It is responsible for the vast majority of FeLV-associated symptoms and often leads to secondary infections that require treatment with bacterial, viral and protozoal pathogens as well as fungal infections.
These infections can, due to the immune deficiency, cause more severe symptoms than in healthy cats. These include B. Infections with Mycoplasma haemofelis or Cryptococci . Infections that are normally symptom-free in healthy cats, such as infection with Toxoplasma gondii , can cause severe symptoms in immunocompromised FeLV-infected cats. In addition, infections with opportunistic pathogens such as B. Salmonella , which could not cause infection in healthy cats. Treatment of the secondary infection is often more tedious and requires more aggressive therapy than in healthy cats.
A further consequence of the weakening of the immune response is a reduced tumor defense, so that progressively infected cats have an increased risk of developing tumors not directly induced by the FeLV virus.
Immune-Mediated Diseases
Although the humoral immune response to specific stimulation decreases in the course of a FeLV infection, some FeLV-infected cats also show unspecifically increased levels of immunoglobulin G (IgG) and immunoglobulin M (IgM) . The immunological dysregulation associated with FeLV infection, especially the disruption of T suppressor cell activity, can lead to an excessive antibody response to the chronic persistent infections. Since the antibodies that are formed cannot neutralize, antigen-antibody complexes are formed , which are deposited in the narrow capillary bed and there lead to vascular inflammation. As a clinical consequence, glomerulonephritis , polyarthritis or uveitis with deposits of immune complexes in the iris and the ciliary bodies can occur. Immune-mediated hemolytic anemia (IHA) is also less common.
Stomatitis
Chronic ulcerative-proliferative gingivostomatitis may develop in some infected cats . Histologically, immigrated plasma cells and lymphocytes can be detected in the mucous membrane , accompanied by neutrophilic and eosinophilic inflammation of varying degrees . The lesions are very painful, which is why the affected animals often refuse to eat and lose weight. Often teeth also fall out.
The causes of FeLV-associated gingivostomatitis are unclear. Here, too, the histological findings suggest the involvement of an excessive immune response to the chronic antigen stimulation or an immunological dysregulation. A more or less strong involvement of secondary infections such as B. with caliciviruses is considered likely.
Neuropathies
FeLV-infected cats can develop neurological symptoms, most of which are a result of lymphoma or lymphocytic infiltration of the brain or spinal cord, causing compression of the surrounding tissue. However, since neurological symptoms were also observed in cats in which no tumorous changes in the central nervous system could be detected in a later section , it is assumed that the FeLV can also have a direct neurotoxic effect in the case of infections of nerve cells. It has been shown that glycoproteins in the virus envelope can increase the intracellular level of free calcium in the nerve cells, leading to neuronal cell death. Degenerative changes in the white matter with a dilatation of the myelin sheaths as well as swollen axons in the area of the spinal cord and the brain stem could be detected microscopically.
The most commonly reported neurological symptoms include abnormal vocalizations, hyperesthesia, and paresis that worsened to paralysis . Some cats have anisocoria , mydriasis , central blindness, or Horner's syndrome , while others have urinary incontinence . Symptoms usually start out mildly and get worse as the disease progresses.
Fertility disorders
In pregnant cats, FelV viraemia leads to embryonic fetal death, stillbirths or the birth of viraemic kittens, which take care of and die after a short time ( fading kitten syndrome ). The virus is usually not transmitted to the kittens by regressively infected cats, but it is possible that individual kittens in a litter later develop viraemia. In these cases, infection occurs through breast milk from individual mammary complexes in which the virus can persist. By activating the glandular tissue towards the end of pregnancy, virus replication can be reactivated, so that infectious viruses are excreted in the breast milk of the affected mammary complex.
diagnosis
Verification procedure
Various methods are available for the diagnostic detection of FeLV infection. In addition to the detection of virus protein, the polymerase chain reaction can be used to detect both viral DNA and viral RNA in blood samples or in the bone marrow. An immunofluorescence assay (IFA) and antibody detection are also available. When interpreting the findings obtained using the various methods, it must always be taken into account that FeLV infection can take very different forms and that even regressively infected cats cannot completely eliminate the virus from the body.
| Course of infection | p27 antigen detection from blood | viral RNA-PCR from blood | proviral DNA-PCR from blood | Virus shedding | Development of FeLV-associated diseases |
|---|---|---|---|---|---|
| progressive infection | positive | positive | positive | positive | probably |
| regressive infection | negative or transient | negative or transient | positive | negative | unlikely |
| abortive infection | negative | negative | negative | negative | unlikely |
| focal infection | negative | negative | negative or positive | variable | unlikely |
Since positive evidence of FeLV infection can have consequences with regard to the keeping of the affected cats (need for separation) as well as for the treatment of clinical diseases up to the decision for euthanasia, every positive result of a screening should be carried out -Tests are verified by a test repetition. This is especially true for clinically normal animals whose medical history suggests only a low risk of infection, since the probability of false-positive test results is high here.
The isolation of the feline leukemia virus, which takes place with the help of a cell culture, is considered the diagnostic golden standard. The isolation can take place from infected cells of the blood or tissue samples. This method is mainly used in scientific studies to detect FeLV infection. However, due to the high effort involved, this test is not used in routine veterinary diagnostics.
For the diagnosis of FeLV infection, the detection of the soluble viral capsid protein p27 in the blood with the help of an ELISA is routinely used as a screening test (so-called p27 antigen test). For this examination, rapid tests from various manufacturers are available for use in veterinary practices. All laboratories specializing in veterinary medicine also offer detection of the p27 antigen from blood samples. In principle, the p27 antigen can also be detected in saliva or tear fluid. However, since the virus is only excreted intermittently in some animals, testing a blood sample is preferable.
In areas with a low prevalence, the informative value of a positive test result is lower, with most test systems the positive predictive value is around 80%. Therefore, every positive test should be checked. Due to the high sensitivity of the screening tests and the overall low prevalence of FeLV infection, negative test results can be viewed as very reliable (high negative predictive value ).
The detection of the p27 antigen correlates well with the presence of a viraemia. Most cats that become infected with FeLV will respond positively to antigen detection within 2 to 3 weeks after infection. In the case of a regressive infection, the animals become negative for p27 within 2 to 8 more weeks, in rare cases this can take months. In progressively infected cats, virus antigen can be detected in the blood even a long time after infection. A positive test should therefore be repeated after approx. 6 weeks in order to be able to differentiate between a regressive and a progressive infection. With a second positive result, a third test can be carried out after a further 10 weeks. Animals that still respond positively are very likely to be persistently viraemic.
Cell-bound DNA (provirus) can be detected in blood samples, bone marrow biopsies and aspirates as well as tissue samples via a polymerase chain reaction . Regressively infected cats react negatively in the p27 antigen detection, since no virus replication takes place in them and therefore no viral protein circulates in the blood. In these animals, however, FeLV provirus DNA can be detected in the cells of the blood using the PCR.
PCR can also be used to detect viral RNA in samples from infected cats. Whole blood, serum , plasma , saliva or feces can be used as test material. This method can also be used to detect and quantify free virus that is not bound to the host cell. The result of the DNA-PCR and the RNA-PCR do not always have to match, so many cats that have been able to overcome a viraemia react negatively in the p27 detection and in the RNA-PCR, since there is no longer any virus in the blood. However, since the FeLV genome was integrated into the genome of infected host cells as a provirus, the DNA-PCR was positive.
Granulocytes, lymphocytes, and platelets from viremic cats contain the Gag protein. This can be detected in smears from the blood or bone marrow using an indirect immunofluorescence test (IFA) . Although this method offers a relatively low sensitivity, cats that react positively to the IFA are also very likely to have viraemia. However, in cats with leukopenia or in which only some of the peripheral leukocytes are infected, the IFA can be false negative despite the presence of viraemia.
The IFA only reacts positively after the infection of the bone marrow and during the second viraemia, since only then does the virus bound to the blood cells enter the bloodstream. Since this is an advanced stage of the infection, it is unlikely that cats that test positive at IFA will later react negatively because they have developed a regressive infection.
Immunocompetent cats develop high levels of antibodies for life after surviving viraemia. However, since many animals also produce antibodies against endogenous FeLV in the cat genome, detection of antibodies for the diagnosis of FeLV infection has so far only been of limited use. However, a test method for the detection of antibodies against the p15 (E) protein has now been developed, with which antibodies that have been formed due to an existing FeLV infection can be reliably differentiated from antibodies against endogenous FeLV. However, this method has not yet been used in routine diagnostics.
As a rule, individual animals are tested with the aid of the various test methods available. For screening tests in larger groups of cats, such as stray groups, animal shelters or multi-cat households, samples can be pooled for an RNA-PCR test. The RNA-PCR is so sensitive that a single infected cat can be detected in a pool of up to 30 samples.
Differential diagnosis
Since the FeLV infection itself is associated with a large number of symptoms and the induced immunodeficiency also favors numerous different secondary infections, the possible clinical picture is extremely variable. The induced tumors cause different symptoms depending on their location. For this reason, the diagnostic work-up and differential diagnosis of FeLV infection are of particular importance. Depending on the symptoms present, other neoplastic diseases, the immunodeficiency syndrome of cats (FIV infection, cat enaids), feline infectious peritonitis (FIP), feline infectious anemia (hemobartonellosis) and other infectious diseases must be excluded.
It is generally recommended that all cats with symptoms that may be related to FeLV infection be tested for p27 antigen. This also applies to sick cats for which a test for FeLV with negative results was carried out a long time ago, if it cannot be ruled out with certainty that contact with the virus has taken place in the meantime. Even in the case of symptoms for which an infection can be proven as the cause, it must be taken into account that a FeLV infection with accompanying immunodeficiency is present and can promote the development of other infections. Especially cats with recurrent abscesses of the subcutaneous tissue or inflammations in the oral cavity should therefore be tested for the presence of FeLV infection.
therapy
One is no therapy that can completely remove the virus from the body in a progressively infected cat. Even with regressively infected cats that carry proviruses in their body cells, the virus cannot be eliminated from the body. For this reason, there is no causal therapy for FeLV infection. The treatment of sick FeLV-infected cats is therefore primarily aimed at symptomatic treatment of the symptoms that occur in each case, with the aim of trying to alleviate the suffering of the animals ( palliative therapy ).
The options for antiviral chemotherapy are receiving increasing attention in veterinary medicine, but their use is not yet widespread. With various active ingredients used in human medicine, especially for HIV therapy, it was possible to inhibit FeLV replication in cats. With the active ingredient azidothymidine (AZT) used in human medicine to combat retroviruses, the virus load in the plasma of cats could be reduced and both the immunological and clinical condition improved, so that an improvement in the quality of life and an increase in life expectancy were achieved were. The administration of the active ingredient raltegravir also inhibited virus replication, so that within a week this therapy achieved a significant reduction in the virus load in the blood. However, none of these agents succeed in completely suppressing persistent viraemia. The active ingredients must therefore be administered over a longer period of time in order to achieve a low virus load in the long term and to prevent or delay the onset of clinical symptoms. Although the drugs are often well tolerated by cats, long-term use is often associated with significant side effects.
Cats diagnosed with progressive FeLV infection with persistent viraemia but showing no clinical symptoms do not require drug treatment. However, they should be kept strictly as indoor cats, as this can reduce the risk of secondary infections. At the same time, this is the only way to prevent them from infecting other cats. These cats should be given the usual routine vaccinations to protect the immunocompromised animals from infection. However, it must be taken into account that these animals may react to the vaccination with an insufficient immune response due to a possibly existing weakening of the immune system. Therefore, the vaccination protection of FeLV-infected cats is not complete and cannot be compared to that of healthy animals, so that shorter vaccination intervals than for healthy animals (e.g. every 6 instead of every 12 months) should be considered. In general, an inactivated vaccine should be given preference over live vaccines for these animals, since with a weakened immune system it cannot be ruled out that even the weakened vaccine strains may develop pathological potential.
Owners of FeLV-positive cats should also refrain from giving them unheated food, as this carries the risk of transmitting bacterial or parasitic infections. The health status of FeLV-infected asymptomatic cats should be checked regularly. It is therefore advisable to have a blood count, a blood chemistry test and a urinalysis carried out at intervals of 6 to 12 months in order to identify and treat health problems at an early stage.
FeLV-infected cats showing symptoms of disease should undergo thorough diagnosis and treatment immediately to allow for early intervention. The first thing to do is to clarify which disease is the primary cause of the symptoms. Not all symptoms that occur must be directly related to the FeLV infection. Careful and comprehensive diagnostics are therefore important in order to be able to treat the underlying disease in a targeted and consistent manner.
Due to the immune weakness that is usually present, longer or more aggressive therapy is often necessary than with FeLV-negative animals, e.g. B. in antibiotic treatment. Corticosteroids, other immunosuppressants, or drugs that can lead to bone marrow suppression should generally not be administered except for the treatment of FeLV-associated tumors or immune-mediated diseases.
In FeLV-positive cats suffering from anemia, the causal cause must first be clarified. The immune deficiency can cause secondary infections that cause anemia (e.g. Mycoplasma spp.). If this is the case, these infections must be treated specifically. If the anemia is severe, a blood transfusion can be performed. If the anemia is FeLV-induced, treatment with glucocorticoids can be attempted , as some of the anemias associated with FeLV are immune-mediated and these cats respond well to therapy. Glucocorticoids should only be used in FeLV-positive cats if strictly indicated, as the immunosuppressive effect can contribute to a further weakening of the immune system.
For the treatment of feline lymphoma, there are established treatment protocols in veterinary medicine using chemotherapeutic drugs . The COP scheme ( cyclophosphamide , vincristine , prednisolone ) and the active ingredient chlorambucil have proven successful . In some cases, the tumors respond well to treatment. Although chemotherapy can often achieve remission of a lymphoma, it must be clear that even successful chemotherapy has no influence on persistent viraemia. The medium to long-term prognosis for FeLV-positive cats is therefore less favorable than for FeLV-negative cats with malignant lymphoma.
Even in FeLV-infected cats with neurological symptoms, the cause of the symptoms must first be clarified. In addition to the directly toxic effects of FeLV replication, the neurological symptoms can also be based on a lymphoma of the CNS, secondary infections (for example with Cryptococcus ssp.) Or toxoplasmosis. Infectious diseases must be treated directly in this case. If no other causal disease can be determined and it is assumed that the neurological symptoms are caused directly by the FeLV, a treatment attempt with the antiviral azidothymidine (AZT) can be carried out.
In FeLV-infected cats that suffer from recurrent infections due to the immunodeficiency associated with the infection, these infections must be treated consistently in order to ensure a good quality of life. Treatment with recombinant feline interferon omega as an immunomodulator is possible.
forecast
The overall prognosis for persistent viremic cats is to be assessed as unfavorable. In the overwhelming majority, clinical symptoms develop within months and the animals have a reduced life expectancy. Studies have shown that around 50% of progressively infected cats die within 2 years and 80% within 3 years of diagnosis.
However, some cats can live in good health with persistent viraemia for many years before they develop symptoms. The death rate can be significantly reduced through good care and maintenance by the owner and housing, which can reduce the risk of secondary diseases. In particular, early and consistent treatment of secondary infections can contribute to an increase in life expectancy with a good quality of life.
The decision of whether to euthanize or treat a FeLV-infected cat should never be based on the presence of FeLV infection alone. Euthanasia is not indicated for viremic but symptomless cats, as it can take years for the disease to break out. However, it must be strictly ensured that these animals have no contact with FeLV-negative animals in order to avoid transmission of the virus. If a separation is not possible, e.g. E.g. in the case of stray cats that cannot be accustomed to exclusive housing, euthanasia should be considered to prevent the spread of FeLV infection.
prophylaxis
vaccination
Vaccines against FeLV have been commercially available since 1985. Adjuvanted preparations from inactivated virus, adjuvanted preparations from recombinant virus surface protein and an adjuvant-free recombinant vector vaccine based on the canarypox virus are permitted. The vaccination effect achieved is based on cellular immunity, which quickly leads to the formation of neutralizing antibodies as soon as cats come into contact with the naturally occurring FeLV field virus. After the primary vaccination course, all vaccines offer reliable protection against the development of persistent viraemia and against the development of clinical symptoms of FeLV infection. However, none of the available vaccines reliably induce sterile immunity, so that an infection cannot be prevented even in vaccinated cats, which can safely overcome this thanks to the vaccination protection. This means that even in vaccinated cats after contact with FeLV provirus DNA can be detected in the blood and mostly also viral RNA in the plasma, but only in very low concentrations compared to persistent viraemic cats, so that they are not clinically relevant and these cats can be regarded as safely protected against the development of FeLV-associated diseases with normal infection pressure.
Both the German-speaking national and international guidelines for vaccinating small animals classify the vaccination against the feline leukemia virus as a non-core vaccination. This means that FeLV vaccination is not generally recommended for every cat to be vaccinated, but should only be carried out after weighing up the individual risk of infection. The vaccination is recommended for cats that are at risk of infection because they may have contact with cats with an unexplained infection status. This is e.g. B. for cats with free access, for breeding and exhibition animals or animals that are taken into an animal shelter, the case. The FeLV vaccination should only be avoided in cases in which the possibility of exposure to FeLV can be completely ruled out. Geographical differences in FeLV prevalence and the fact that the conditions in which the cat is kept, and thus the risk of FeLV exposure, change over time, e.g. B. by moving, should be considered when deciding whether a cat should be vaccinated against FeLV. In general, the FeLV vaccination offers sufficient protection against the potentially fatal consequences of an infection, which must be weighed against the low risk of side effects.
Young cats can be vaccinated against FeLV from 8 weeks of age. For primary vaccination, two injections are given 3 to 4 weeks apart. With another injection after a year, the basic immunization is completed. Most of the available vaccines are approved for a one-year vaccination interval. Since the susceptibility to the virus decreases with increasing age, for cats that are older than 3 years, after a successful basic vaccination, revaccinations every 2 to 3 years are considered sufficient. Conversely, young cats are particularly susceptible to the infection and have a higher risk of developing a progressive infection, which is why they should be well protected by vaccination, especially in the first few years of life.
For cats whose FeLV vaccination status is unknown, testing for FeLV antigen in the blood is recommended before the first vaccination. Vaccination does not make sense for cats that were already infected before the vaccination, as the vaccination cannot suppress a progressive infection and therefore does not protect against the development of FeLV-associated symptoms.
Avoiding infection
In addition to vaccination, avoiding infection is an important prophylactic measure. Progressively infected cats should be strictly separated from uninfected animals. They should be kept as indoor cats to prevent the virus from spreading in the vicinity. Keeping cats separately and without access protects FeLV-infected cats, which often suffer from a weakened immune system, from contact with infectious agents.
If a cat in a multi-cat household tests positive for FeLV, all other cats should also be tested. All animals tested positive must be strictly separated from cats tested negative in order to prevent further spread of the infection. Vaccination of the negatively tested animals offers a certain protection, but cannot guarantee that the vaccinated animals will not develop a progressive infection if there is a very high infection pressure from several virus-shedding animals in the immediate vicinity.
Both male and female progressively infected cats should be neutered to minimize the risk of virus transmission to other animals through turf wars and mating behavior. The surgical intervention of castration is usually very well tolerated even by progressively infected animals. If the standard hygiene regulations are complied with, there is no risk of passing the infection to other animals in the veterinary practice.
When adding new cats with unknown FeLV status to an existing FeLV-negative animal group, e.g. B. in animal shelters, animal breeding or private households, it is recommended to initially keep the new animals in quarantine for at least 3 weeks and then to carry out a test for FeLV antigen before there is direct contact between the animals. Animals that react negatively to the antigen test should be kept in quarantine before the test has to be repeated 6 weeks later, as it takes 4 to 6 weeks for antigen to be detectable in the blood after an infection has taken place. Positive-reacting cats should only be kept in groups with other positive-tested animals. Many animal shelters arrange for FeLV-positive cats to be kept in solitary confinement without access to and contact with FeLV negative cats. However, owners must be thoroughly educated about the infection and the risks to other cats so that contact with other cats is safely avoided. It is recommended that FeLV-positive cats be euthanized in animal shelters.
Since direct contact with the exchange of body fluids, especially saliva, is necessary for transmission, infected cats can e.g. B. in an animal shelter or in a stationary stay in a veterinary clinic, be kept in the same room as non-infected cats, as long as there is direct physical contact between the animals, for. B. is prevented by placing them in cages and taking hygienic measures into account when caring for the animals. However, care must be taken to ensure that the FeLV-infected animals are not exposed to an increased risk of secondary infections from the other animals.
The FeLV infection can also be transmitted through a blood transfusion, which is why the guidelines for blood transfusion in cats provide for a test of the donor animal for FeLV infection. Since provirus-positive blood cells can be transferred during a blood transfusion, a PCR test for provirus DNA is recommended, as regressively infected animals cannot be identified by an antigen test. Testing should be carried out no earlier than three months after the last contact with a potential source of infection in order to avoid false-negative results during the longer incubation period. In acute life-threatening emergencies in which no other donor is available for a short time, the risk of FeLV transmission must be weighed up. In these cases, at least a rapid test for p27 antigen should be performed. The owner of the recipient animal must be informed of the risk of transmission.
The feline leukemia virus is relatively sensitive and quickly loses its infectivity in the environment . It is safely inactivated by all common disinfectants as well as by soap solutions . For this reason, compliance with general hygiene standards in veterinary practices, animal shelters and private households is usually sufficient to avoid indirect transmission of the virus through contaminated objects, such as examination and treatment instruments, surgical instruments, food bowls or even hands.
Web links
Individual evidence
- ↑ a b c d e f g h i j k l m n o p q r s K. Hartmann: 2.2. FeLV-associated tumors. In: Martin Kessler (ed.): Small animal oncology: diagnosis and therapy of tumor diseases in dogs and cats. 3rd, completely revised and expanded edition, Enke-Verlag, Stuttgart 2012, ISBN 3-8263-3236-9 , pp. 17-19
- ↑ E. Dahme, E. Weiss: 2.5. Leukosis. In: Outline of the special pathological anatomy of domestic animals. 6th, completely revised edition, Thieme Verlagsgruppe, Stuttgart 2006, ISBN 978-3-8304-1048-5 , doi: 10.1055 / b-002-8317 , p. 44
- ↑ AW Philbey: Viruses and cancer, cats and cattle: a tribute to Bill Jarrett. In: The Veterinary Journal. (1) 195, 2013 pp. 2–3, PMID 23164956 , doi: 10.1016 / j.tvjl.2012.10.015
- ↑ a b c d e f g h i j k l m n o p q r Julia Beatty: Viral causes of feline lymphoma: Retroviruses and beyond. In: The Veterinary Journal. Vol. 201, (2) 2014 (Special Issue: Feline Infectious Diseases.), Pp. 174–180, PMID 24928422 , doi: 10.1016 / j.tvjl.2014.05.026
- ↑ WFH Jarrett, WB Martin, GW Crighton, RG Dalton, MF Stewart: Leukæmia in the Cat: Transmission Experiments with Leukæmia (Lymphosarcoma). In: Nature . Vol. 202, 1964, pp. 566-567, PMID 14195053 , doi: 10.1038 / 202566a0
- ↑ Jarrett WFH, Crawford EM, Martin WB, Davie FA: A Virus-Like Particle Associated with Leukemia (Lymphosarcoma). In: Nature. Vol. 202, 1964, pp. 567-569; PMID 14195054 , doi: 10.1038 / 202567a0
- ↑ Voisset C, Weiss RA, Griffiths DJ: Human RNA "rumor" viruses: the search for novel human retroviruses in chronic disease . In: Microbiol Mol Biol Rev . tape 72 , no. 1 , March 2008, p. 157-96 , doi : 10.1128 / MMBR.00033-07 (English).
- ↑ Robert Gallo: The Hunt for the Virus . S. Fischer-Verlag, 1991, ISBN 3-10-024404-4 , chap. 8. A single disease with a single cause, p. 190 .
- ^ A b c Brian J. Willett, Margaret J. Hosie: Feline leukaemia virus: Half a century since its discovery. In: The Veterinary Journal. 195 (2013) pp. 16-23, PMID 22867855 , doi: 10.1016 / j.tvjl.2012.07.004
- ↑ a b c d e f g h i K. Möstl, DD Addie, C. Boucraut-Baralon, H. Egberink, T. Frymus, T. Gruffydd-Jones, K. Hartmann, MJ Hosie, A. Lloret, H. Lutz, F. Marsilio, M. Grazia Pennisi, AD Radford, E. Thiry, U. Truyen, MC Horzinek: Something old, something new - Update of the 2009 and 2013 ABCD guidelines on prevention and management of feline infectious diseases. In: Journal of Feline Medicine and Surgery. (7) 17, 2015, pp. 570-582, PMID 26101308 doi: 10.1177 / 1098612X15588448
- ^ A b S. J. O'Brien, JL Troyer, MA Brown, WE Johnson, A. Antunes, ME Roelke, J. Pecon-Slattery: Emerging Viruses in the Felidae: Shifting Paradigms. In: Viruses. (4) 2012, pp. 236-257, PMID 22470834 , doi: 10.3390 / v4020236
- ↑ ML Meli, V. Cattori, F. Martinez, G. Lopez, A. Vargas, MA Simon, I. Zorrilla, A. Munoz, A., F. Palomares, JV Lopez-Bao, JV et al .: Feline leukemia virus and other pathogens as important threats to the survival of the critically endangered Iberian lynx (Lynx pardinus). In: PLoS One (4) 2009, e4744, PMID 19270739 , doi: 10.1371 / journal.pone.0004744
- ↑ a b c d e f g h i j k l m n o p q r s t u v w x y z aa ab ac ad ae af H. Lutz, D. Addie, S. Belák, C. Boucraut-Baralon , H. Egberink, T. Frymus, T. Gruffydd-Jones, K. Hartmann, MJ Hosie, A. Lloret, F. Marsilio, M. Grazia Pennisi, AD Radford, E. Thiry, U. Truyen, MC Horzinek: Feline Leukemia: ABCD Guidelines on Prevention and Management. In: Journal of Feline Medicine and Surgery. Vol. 11, (07) 2009, pp. 565-574, PMID 19481036 , doi: 10.1016 / j.jfms.2009.05.005
- ↑ a b c d e f g h i j k l m n o p q r s t u v w x y z aa ab ac ad ae af ag ah ai aj ak al am K. Hartmann: Clinical Aspects of Feline Retroviruses: A review. In: Viruses. (4) 2012, 4, pp. 2684-2710, PMID 23202500 , doi: 10.3390 / v4112684
- ↑ a b c d e f g h i j k l J. Levy, C. Crawford, K. Hartmann, R. Hofmann-Lehmann RS Little, E. Sundahl, V. Thayer: 2008 American Association of Feline Practitioners' feline retrovirus management guidelines. In: Journal of Feline Medicine and Surgery. Vol. 10, (3) 2008, pp. 300-316, PMID 18455463 , doi: 10.1016 / j.jfms.2008.03.002
- ↑ a b c d e f g h i j k Thomas Vahlenkamp: 2.1. Virus-Associated Oncogenesis - Chapter 2.1.2. Retroviruses. In: Martin Kessler (ed.): Small animal oncology: diagnosis and therapy of tumor diseases in dogs and cats. 3rd, completely revised and expanded edition, Georg Thieme Verlag 2012, ISBN 3-8263-3236-9 , pp. 15-16
- ↑ MC Horzinek, D. Addie, S. Belák, C. Boucraut-Baralon, H. Egberink, T. Frymus, T. Gruffydd-Jones, K. Hartmann, MJ Hosie, A. Lloret, H. Lutz, F. Marsilio , K. Möstl, M. Grazia Pennisi, AD Radford, E. Thiry, U. Truyen: ABCD - Update of the 2009 guidelines on prevention and management of feline infectious diseases In: Journal of Feline Medicine and Surgery Volume 15, Issue 7, 2013, pp. 530-539, PMID 23813810 , doi: 10.1177 / 1098612X13489208
- ↑ a b c d e K. Hartmann: Clinical aspects of feline immunodeficiency and feline leukemia virus infection. In: Veterinary Immunology and Immunopathology. (3-4) 143, 2011, pp. 190-201, PMID 21807418 , doi: 10.1016 / j.vetimm.2011.06.003
- ↑ Table based on: Levy, C. Crawford, K. Hartmann, R. Hofmann-Lehmann RS Little, E. Sundahl, V. Thayer: 2008 American Association of Feline Practitioners' feline retrovirus management guidelines. In: Journal of Feline Medicine and Surgery. Vol. 10, (3) 2008, pp. 300-316, PMID 18455463 , doi: 10.1016 / j.jfms.2008.03.002
- ↑ SN Ettinger: Principles of treatment for feline lymphoma. In: Clinical Techniques in Small Animal Practice. (2) 18, 2003, pp. 98-102, PMID 12831069 , doi: 10.1053 / svms.2003.36623
- ↑ DA Grosenbaugh, V. Frances Duvert, S. Abedi, B. Feil Meier, H. Ru, H. Poulet: Efficacy of a recombinant nonadjuvanted FeLV vaccine and two inactivated FeLV vaccines When subject to consistent virulent FeLV challenge conditions. In: Biologicals. 49 (2017), pp. 76-80, PMID 28734742 , doi: 10.1016 / j.biologicals.2016.10.004
- ↑ a b Standing Vet. Vaccination Commission at the Friedrich-Loeffler-Institut : Vaccination recommendation for cats. In the guideline for the vaccination of small animals. 4th edition, as of February 1, 2019 , p. 14, published on the homepage of the Federal Association of Practicing Veterinarians (bpt), accessed on March 11, 2020
- ↑ Standing Vaccination Commission of the Swiss Association for Small Animal Medicine : Vaccination recommendations of the SVK-ASMPA 2017. , January 2017 edition, published on the homepage of the Swiss Association for Small Animal Medicine SVK + ASMPA, accessed on March 27, 2018
- ↑ Department of Internal Medicine at the Clinic for Small Animals , Department for Small Animals and Horses at the University of Veterinary Medicine Vienna and the Austrian Chamber of Veterinarians: Vaccination guidelines for small animals 2017, as of May 2017 , published on the homepage of the Austrian Chamber of Veterinarians, accessed on March 27, 2018
- ↑ MJ Hosie, DD Addie, C. Boucraut-Baralon, H. Egberink, T. Frymus, T. Gruffydd-Jones, K. Hartmann, MC Horzinek, A. Lloret, H. Lutz, F. Marsilio, M. Grazia Pennisi , AD Radford, E. Thiry, U. Truyen, K. Möstl: Matrix vaccination guidelines - 2015 ABCD recommendations for indoor / outdoor cats, rescue shelter cats and breeding catteries. In: Journal of Feline Medicine and Surgery. Vol. 17, 2015, pp. 583-587, PMID 26101309 , doi: 10.1177 / 1098612X15590732
- ↑ MJ Day, MC Horzinek, RD Schultz, RA Squires: Guidelines for the Vaccination of dogs and cats compiled by the Vaccination Guidelines Group (VGG) of the World Small Animal Association (WSAVA). In: Journal of Small Animal Practice. Vol (1) 57 2016, pp. E1-E45, PMID 26780853 , doi: 10.1111 / jsap.12431
- ^ A. Scherk, RB Ford, RM Gaskell, K. Hartmann, KF Hurley, MR Lappin, JK Levy, SE Little, SK Nordone, AH Sparkes: 2013 AAFP Feline Vaccination Advisory Panel Report. In: Journal of Feline Medicine and Surgery. (9) 15, 2013, pp. 785-808, PMID 23966005 doi: 10.1177 / 1098612X13500429
- ↑ M. Grazia Pennisi, K. Hartmann, DD Addie, H. Lutz, T. Gruffydd-Jones, C. Boucraut-Baralon, H. Egberink, T. Frymus, MC Horzinek, MJ Hosie, A. Lloret, F. Marsilio , AD Radford, E. Thiry, U. Truyen, K. Möstl: Blood transfusion in cats - ABCD guidelines for minimizing risks of infectious iatrogenic complications. In: Journal of Feline Medicine and Surgery. 17 (2015), pp. 588-593, PMID 26101310 , doi: 10.1177 / 1098612X15588449









