Turquoise (mineral)
| turquoise | |
|---|---|
|
Turquoise tuber from Arizona, United States Size: 7 × 5 × 5 cm |
|
| General and classification | |
| chemical formula | Cu (Al, Fe) 6 (PO 4 ) 4 (OH) 8 · 4 H 2 O |
|
Mineral class (and possibly department) |
Water-containing phosphates with foreign anions |
|
System no. to Strunz and to Dana |
8.DD.15 ( 8th edition : VII / D.15) 42.09.03.01 |
| Similar minerals | Amazonite , chrysocolla , hemimorphite , lazulite , serpentine , variscite |
| Crystallographic Data | |
| Crystal system | triclinic |
| Crystal class ; symbol | triclinic pinacoidal; 1 |
| Space group | P 1 (No. 2) |
| Lattice parameters |
a = 7.410 Å ; b = 7.633 Å; c = 9.904 Å, α = 68.42 °; β = 69.65 °; γ = 65.05 ° Please complete the source as an individual reference |
| Formula units | Z = 1 Please complete the source as an individual reference |
| Frequent crystal faces | {100}, {010}, {001} |
| Twinning | no |
| Physical Properties | |
| Mohs hardness | 5 to 6 |
| Density (g / cm 3 ) | 2.6 to 2.9 |
| Cleavage | good after {010}, perfect after {001} |
| Break ; Tenacity | shell-like, uneven |
| colour | blue, blue-green, green |
| Line color | greenish white |
| transparency | transparent to opaque |
| shine | Wax gloss, matt |
| Crystal optics | |
| Refractive indices |
n α = 1.610 n β = 1.615 n γ = 1.650 |
| Birefringence | δ = 0.040 |
| Optical character | biaxial positive |
| Axis angle | 2V = 40 ° |
| Pleochroism | weak, colorless-light blue-light green |
| Other properties | |
| Chemical behavior | soluble in heated hydrochloric acid |
| Special features | green fluorescence with long-wave UV light |
The mineral turquoise is a rather rarely occurring, water-containing copper - aluminum - phosphate from the mineral class of " phosphates , arsenates and vanadates " with the chemical composition CuAl 6 (PO 4 ) 4 (OH) 8 · 4H 2 O. Instead of aluminum, can Iron can be incorporated into the crystal structure as Fe 3+ ( Diadochie ), which is why the chemical formula is often given as Cu (Al, Fe) 6 (PO 4 ) 4 (OH) 8 · 4H 2 O.
Turquoise crystallizes in the triclinic crystal system and gives its name to a group of minerals with the same structure but different composition, the turquoise group with the other members Faustite , Chalkosiderite , Aheylite and Planerite .
In nature, turquoise usually forms grape-shaped or earthy, massive mineral aggregates . Crystals visible to the naked eye are very rare and then only a few millimeters in size with a prismatic to needle-like habit . The color turquoise owes its name to its characteristic blue-green color .
Turquoise is only processed into gemstones .
etymology
As an early name, the ancient Greek καλάϊνος kalláïnos “shimmering blue and green” can be assumed (from Pliny , Naturalis historia ). The Latin callaina is derived from this . Gotthelf Fischer von Waldheim used the term Kallait for turquoise around 1806 ; these days, however, it is rarely used.
Around the beginning of the 13th century, the French name turkoys came up, which changed from the early 15th century to the name pierre turquoise and translated means “Turkish stone”. However, this word creation is based on a misunderstanding, because at that time turquoise was only imported into Turkey from the area of today's Iran and traded there. Returning crusaders finally made him known in Europe.
Further synonyms for turquoise are Bisbee Blue - after its place of discovery Bisbee - as well as Chalchite or Chalchuit .
The misleading name Eilat stone , on the other hand, stands for chrysocolla .
classification
Both the mineral classification according to Strunz and the classification according to Dana , which is used in the English-speaking world , place turquoise in the mineral class of "phosphates, arsenates and vanadates".
In the outdated, but partly still in use 8th edition of the Strunz system , the turquoise belonged to the division of " water-containing phosphates with foreign anions ", where it named the "turquoise group" with the system no. VII / D.15 and the other members aheylite , chalcosiderite , Faustite and planerite .
The 9th edition of the mineral classification according to Strunz , which has been valid since 2001, has retained the department and group name, but the department is now more precisely subdivided according to the size of the cations involved and the molar ratio between foreign anion and sulfate, arsenate or vanadate complex. According to its composition, the turquoise belongs to the subdivision “With only medium-sized cations; (OH etc.): RO 4 = 2: 1 "and still forms together with aheylite, chalcosiderite, Faustite and planerite the" turquoise group "with the system no. 8.DD.15 .
In the systematics of minerals according to Dana , which is mainly sorted according to the crystal system , the turquoise is found in the section of " water-containing phosphates etc., with hydroxyl or halogen with (A) 3 (XO 4 ) 2 Z q • x (H 2 O) " and there as a triclinic crystallizing mineral in the "turquoise group" with the system no. 42.09.03 .
Crystal structure
| Crystallographic Data | ||
|---|---|---|
 Unit cell of turquoise |
||
| Crystal system | triclinic | |
| Space group | P 1 | |
|
Lattice parameters |
a = 7.410 Å b = 7.633 Å c = 9.904 Å |
α = 68.42 ° β = 69.65 ° γ = 65.05 ° |
| Number (Z) of the formula units | Z = 1 | |
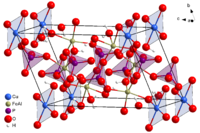
Measured against the millennia in which turquoise was known worldwide and valued as a gem, its crystal structure was explained unusually late. Such structural analyzes are based on the evaluation of X-ray diffraction experiments on single crystals of sufficient size and quality. Turquoise, however, has always been known only in the form of earthy, cryptocrystalline masses. Turquoise monocrystals were first described in 1912 from an occurrence in Virginia (USA) and it was not until 1965 that the turquoise structure with monocrystals from this location could be fully elucidated.
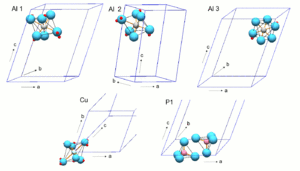
Turquoise crystallizes in the triclinic crystal system in space group P 1 (space group no. 2) . The only element of symmetry is an inversion center, which multiplies the atoms by point reflection. Since copper coincides with the center of inversion, it is the only particle in the chemical formula that appears only once. In crystallography , the collapse of particles with an element of symmetry is called a special position . All other atoms are in a symmetry-free, so-called general position . The lattice parameters of the unit cell are given in the table.
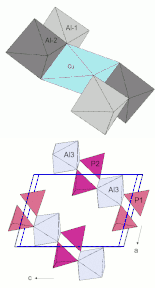
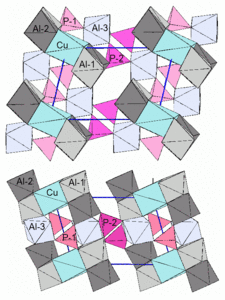
The cations are four- and six-fold coordinated by oxygen in the crystal structure . The two crystallographically different phosphorus cations (P 5+ ) in the crystal lattice are coordinated as the only particles by only four oxygen atoms in the form of a tetrahedron . These [PO 4 ] 3− tetrahedra are not connected to one another, but are isolated in the structure. Each phosphorus ion is connected to two Al ions on the Al-3 position via two oxygen atoms, with one Al ion on Al-1 and another on Al-2.
Aluminum (Al 3+ ) and the low content of Fe 3+ ions are in three different positions, each octahedral surrounded by six oxygen atoms. Al at positions Al-1 and Al-2 is coordinated by two oxygen atoms, three OH groups and one H 2 O molecule. Al at position Al-3 is surrounded by four oxygen atoms and two OH groups.
Copper is located in an inversion center on the corners of the unit cell and is surrounded by four OH groups and two H 2 O molecules in a distorted octahedral manner . This strongly distorted [CuO 6 ] octahedron is connected to four [AlO 6 ] octahedra via common edges , two of which in turn are connected to one another via a common edge. This group of five linked octahedra within the crystal structure can be referred to as a cluster and, for ease of description of the structure, can be viewed as a building unit.
The clusters of edge- sharing [CuO 6 ] and [AlO 6 ] octahedra are connected to one another via a further AlO 6 octahedron and the PO 4 tetrahedron. The connection of this third [AlO 6 ] octahedron with the [PO 4 ] tetrahedra and the Cu-Al octahedron clusters takes place via common corners, that is, common oxygen atoms.
For the sake of simplicity, the structure diagrams do not show the atoms and their bonds, but the coordination polyhedra (tetrahedron and octahedron). The cations Cu 2+ , Al 3+ , P 5+ are located approximately in the center of the polyhedra and the oxygen atoms bound to them are in the polyhedron corners. The hydrogens of the OH groups and H 2 O molecules are also not shown . Their oxygen atoms contribute to the octahedral coordination of copper and aluminum. The blue lines in the second figure mark the edges of the unit cell.
properties
Pure turquoise reach a maximum Mohs hardness of just under 6 and are therefore about as hard as window glass . According to the respective formation conditions, the turquoise has a more or less large porosity . The more porous the stone, the lower its relative density, which fluctuates between 2.6 and 2.9 g / cm³, and the lower its hardness. The size of the stone also affects these properties. The line color is a pale bluish white and its break point is mother-of-pearl (conchoid) with a waxy sheen. Despite its low hardness compared to other gemstones, it can be easily polished.
Turquoises are rarely of a pure, blue-green color. Much more often they are found strewn with small spots or interspersed with brown, gray or black, cobweb-like veins, the so-called matrix (corresponding to turquoise matrix or matrix turquoise). This consists either of other minerals such as pyrite or of secondary rocks such as limonite and others.
The index of refraction , measured under sodium light (589.3 nm wavelength ), is approximately 1.61 to 1.62, this value being based on a single measurement by a refractometer . The polycrystalline structure of turquoise makes it difficult to determine a uniform value for the entire mineral. For individual crystals, values from 1.61 to 1.65 ( birefringence 0.040 biaxially positive) have also been measured. An absorption spectrum can be obtained using a manual spectroscope . Good results are obtained with strongly reflected light. Turquoise fluoresces green, yellow or light blue under long-wave UV light. It is inactive under short-wave UV light or X-rays.
Hydrochloric acid only attacks turquoise when it is heated. Organic acids such as formic, citric or acetic acid, on the other hand, affect all minerals of the turquoise group. Potash lye also decomposes the turquoise. The mineral does not melt in front of the blowtorch , but breaks down to a black powder with crackling noises between 200 and 600 ° C.
Varieties
Both henwoodite (first described by Collins in 1876) and rashleighite ( rashleigite , iron turquoise , first described in 1948 by Russel) are assigned to turquoise as a variety with a low iron content. Other sources consider the name henwoodite to be a synonym for turquoise.
Between the predominantly blue turquoise and the predominantly green chalcosiderite there is a gapless series of mixed crystals , the aluminum-rich compounds being assigned to the turquoise and the iron-emphasized compositions to the chalcosiderite. The color spectrum is therefore as diverse as the properties of the mineral - it ranges from white to light blue to deep blue and can be both green-blue and yellow-blue. The blue color is attributed to the idiochromatic copper, while the green is the result of the addition of the iron that has replaced the aluminum . Other small amounts of calcium , for example, can also cause the color to vary.
Due to dehydration (drying out), which takes place outside the mine shortly after the mineral is broken down, the turquoise loses its color and becomes lighter.
Agapit ( Agaphit ) and Johnit contrast designations are for turquoises with glassy surface.
Education and Locations
Turquoise is a typical secondary mineral . It forms relatively close to the surface up to about 30 to 40 meters below the surface during the weathering of copper-containing, aluminum-rich rocks . In the arid zones of the earth, particularly favorable educational conditions seem to prevail. The copper can come either from copper sulfides such as chalcopyrite (copper pyrites) or from carbonates such as azurite and malachite . The aluminum mostly comes from feldspars . Phosphate is supplied via solutions containing phosphate or comes directly from phosphate minerals such as apatite that are present in the rock . Turquoise pseudomorphoses of feldspar, the copper mineral chrysocolla and apatite are therefore rarely found .
In general, turquoise is deposited in the cavities and crevices of weathered igneous rocks , often together with limonite and other iron oxides, occasionally with alunite . If the bedrock is penetrated by silica during the formation of the turquoise , this ensures natural stabilization in the mineral .
Turquoise is almost always cryptocrystalline , massive and does not take on a regular shape. Crystals are very rare even at the microscopic level, but then short prismatic. Also stalaktitartige forms were found. Turquoise rarely even replaces fossil bones and teeth, which are essentially made up of apatite. Odontolite (fossil bones or ivory ) has so far been assumed to be replaced by turquoise or similar phosphate minerals such as the iron phosphate vivianite . Turquoise twins have not yet been found.
Turquoise is one of the first minerals that mined mined , but were of the old mines are now only a few in operation. They are operated seasonally and on a small scale, often with little or no technical assistance, as turquoise is relatively rare. However, in large copper mines, especially in the United States, turquoise is often discovered as a by-product.
The most important sites from a historical and quantitative point of view are likely to be those in the southwestern USA, Mexico, Sinai, Iran, China and southeastern Australia. A few sites are also known in Europe and Africa.
Iran
More than 2000 years ago, Persia was one of the most important suppliers of fine turquoise. Iran has been one of the main mining and trading areas for centuries, and it is from here that the first turquoise probably came to Europe . The most beautiful specimens can still be found there to this day.
The deposits are concentrated on the area around the 2012 m high mountain Ali-Mersai in the city Neyschabur in the region Khorasan . Turquoise is found there in weathered trachyte , both between limonite and sandstone layers , and in the rubble at the foot of the mountain. The mines of Ali-Mersai and the Sinai Peninsula are the oldest known deposits.
Iranian turquoise is often used as a substitute for feldspar . Although it is mostly provided with whitish spots, it is often preferred to the turquoise from other places because of its color and hardness.
Sinai Peninsula
The ancient Egyptians had been mining turquoise since pre-dynastic times (around 5500 BC). In this context, the mining in Maghara Wadi on the Sinai Peninsula around 3200 BC is documented. The local Monitu call the area the "Land of Turquoise".
There are six mines in the region, all of which are located in the southwest of the peninsula and cover an area of 650 km². From a historical point of view, Serabit el-Chadim and Wadi Maghareh are the two most important mines as they are considered to be one of the oldest known. Wadi Maghareh is about 4 km from an ancient temple dedicated to the goddess Hathor .
The deposits on the Sinai Peninsula are now considered exhausted and no longer have any economic significance. However, they are still of historical value. Only Bedouins now and then visit the old deposits and mine turquoise there using self-made gunpowder . The small mines are endangered by flash floods during the winter months. They are considered to be in danger of collapsing.
Turquoise is found on the Sinai Peninsula in the sandstone that was originally coated with basalt . It's usually greener than Iranian turquoise, but it is also harder and less brittle. This mineral, often referred to as Egyptian turquoise, is the most translucent of them all. Under the microscope you can see many small dark blue disks in the surface structure; a phenomenon that can only be observed with Sinai turquoise. The Eilat rocks can also be found near Eilat , Israel . It is a mixture of turquoise, malachite, and chrysocolla and is often referred to as Israel's national rock. Regional artists who process Eilat often use it to sell on to tourists.
United States
The southwestern United States is a major source of turquoise. The main deposits are or were Arizona , California ( San Bernardino , Imperial and Inyo ), Colorado (Counties Conejos , El Paso , Lake and Saguache ), New Mexico (Counties Eddy , Grant County , Otero and Santa Fe ) and Nevada . The deposits of California and New Mexico were worked by the natives of America with stone tools before Columbus . Cerrillos (New Mexico) is mentioned as the oldest mine. It was the largest mine in the United States before 1920 and is now as good as exhausted. Today, Apache Canyon is the only mine that still extracts enough turquoise to be able to compete in the market.
In the USA, turquoise is found in corridors or in storage, sometimes in small nuggets . It is not infrequently a by-product of copper mining. It is mostly of poor quality and only rarely is it really good material that can withstand the Iranian turquoise in color and hardness. The high iron content causes a more green or yellow color and the high degree of fragility (lime turquoise) prevents further processing of the untreated turquoise in the jewelry industry. The United States' most valuable finds are made in Arizona, with the beautiful Bisbee Blue being a fine example of the state's natural wealth. Nevada is the second largest turquoise producer in the USA. Over time, around 75 to 100 mines were opened here. Nevada turquoise is known for its "cobweb matrix" caused by an attractive brown or black limonite streak.
In 1912 the first single crystal turquoise was found at Lynch Station, Virginia . The crystals that form above the mother stone are so small that a circumference of a millimeter in diameter is considered large. Until 1980, it was widely believed that these crystals were unique to Virginia, but today at least 27 other locations are known. This turquoise is very popular with collectors.
To increase profit and meet demand, turquoise in the US is mostly treated or improved to a certain extent. The treatment methods range from harmless waxing to more controversial methods such as dyeing or impregnation.
Other locations
China has been one of the smaller turquoise springs for over 3000 years . In the provinces of Hubei (Zhushan) and Yunnan (Yunxian), high quality material, mostly in the form of compact needles, is found in fragile, silicified limestone . Marco Polo also reported on finds in what is now Sichuan . Turquoise is mostly exported in China. But sometimes you can also find turquoise carvings that look very similar to the jade carvings.
In Tibet , where the green turquoise has been valued for a long time, there are also allegedly high-quality deposits in the mountain regions around Derge and Nagari-Khorsum. The existence of these deposits has been questioned by some experts for lack of evidence.
Further turquoise deposits are located in Afghanistan , Australia (Victoria and Queensland ), northern Chile ( Chuquicamata ), Cornwall ( Great Britain ), Saxony , Silesia ( Poland ), Bulgaria and Turkestan .
Use as a gem stone
Turquoise is one of the oldest gemstones and has cast a spell over many peoples since ancient times with its broad range of green-blue tones from delicate pastel to deeply luminous . He adorned the rulers of ancient Egypt , the Aztecs (and probably also the pre-Columbian Mesoamericans ), the Persians and Mesopotamians , as well as nobles in the Indus and partly also in ancient China since the last Shang dynasty . Turquoise came to Europe for the first time with the traders of the Silk Road . During the Biedermeier period , the sky-blue color variations were particularly popular.
In the jewelry industry, however, it has only been processed since the 14th century, as this point in time marked the decline in power of the Catholic Church , which had been using it for church jewelry until then. It was unknown in India until the Mughal times and in Japan until the 18th century. Turquoise was believed to have prophylactic abilities by many of these peoples. It is supposed to change its color depending on the health of the wearer and protect it from evil forces.
Nowadays, in the West, turquoise is mostly found "en cabochon" in silver rings , bracelets made in the Indian style or as roughly crafted pearls in necklaces. Turquoise is also used to a lesser extent by the Zuni for carving in fetishism . Deep blue tones are still considered valuable, but greenish or yellow pieces are very popular with artists. In western culture, turquoise is the traditional birthstone for those born in December.
Egypt
By grave goods is evidence that the ancient Egyptians turquoise since pre-dynastic period used as a gemstone (about 5500 v. Chr.). The most famous pieces, however, are likely to come from Tutankhamun's grave. Especially the death mask of the Pharaoh , generously decorated with turquoise, is well known. The Egyptian goldsmiths also used it for rings , lavish necklaces and pectorals . Turquoise can be found in golden braiding as well as material for amulet carvings ( scarab ), which were further decorated with carnelian , lapis lazuli and later also with colored glass. Turquoise was the gemstone of the Egyptian goddess Hathor and was so coveted by the ancient Egyptians that it was imitated as one of the first gemstones. The ceramic product faience is glazed to produce this lighter fabric . A similar blue "ceramic" from the Bronze Age has been discovered in a grave site in the British Isles .
The French undertook archaeological excavations in Egypt from the mid-19th to the early 20th centuries. These excavations, which also include Tutankhamun's burial chamber , aroused great interest in the western world and influenced the jewelry, architecture and art of the time. Turquoise, which was coveted for its coloring since 1810, became the trademark of the Egyptian Revival pieces.
Asia
In Tibet and Mongolia , cabochons with imported turquoise and coral were and are very often used in the silver and gold jewelry industries. Greener stones are often preferred here. Nowadays these pieces are often made for the western market and are just an inaccurate representation of the original design. The turquoise is roughly polished into irregular cabochons and set in silver.
Aztecs and other American cultures
The Aztecs used turquoise, but also gold , quartz , malachite , gagat , jade , corals and shells to create deterrent and probably ritual objects decorated with mosaics, such as masks, knives and shields. Wood, bones and shells could serve as a substrate for a mosaic and resins , bitumen and wax as adhesives .
In addition to the Aztecs, the Pueblo , Diné and Apaches also valued turquoise as a gemstone. They used it for amulets and the Apaches said they supported forces in archery . Turquoise was also used by these peoples to decorate sculptures, ring-shaped beads and pendants. The Anasazi of the Chaco Canyon and its adjacent areas are said to have become very rich from the turquoise trade. The unique silver jewelry of the Navajo and other Southwest American Indian tribes , however, is more of a modern phenomenon and is attributed to European influences of the 19th century.
Bible
There is a description of the “armor of righteousness” as part of a priestly robe for Aaron in the Book of Exodus in the Luther Bible (Exodus 28: 15–30). The breastplate ( choschen ) attached to the efod (priest's apron ) was decorated with twelve precious stones set in gold and arranged in four rows. The name of one of the Twelve Tribes of Israel was engraved on each gemstone . Various scholars have translated the first and second stones of the third row as turquoise, but others believe that the stones are jacinth ( zircon , hyacinth) and agate . However, scholars disagree as to which stone corresponds to which tribe.
Persia
In Persia , turquoise has been the national gemstone for thousands of years. In Persian mythology, only the king and his sub-kings were allowed to own the throne and crown with turquoise. Turquoise was (later) used to decorate various everyday objects ( turbans ), mosques and other important buildings such as the Madrassa-I Shah Hussein Mosque in Isfahan . During the Mughal Empire, the Persian style and use of turquoise also came to India, where it can be admired in fine gold jewelry (along with rubies and diamonds ) and buildings such as the Taj Mahal . Persian turquoise was often engraved with Arabic script and then decorated with gold.
Appreciation and care
The value of turquoise as a gemstone is generally determined by the intensity of the color, with a deep sky blue being the most desirable. The greener, lighter or more speckled the stone, the less it is worth. In the East Asian countries, however, as mentioned, a green color is preferred.
Regardless of the color of the stone, it should not be too soft or chalky, even if it has been post-treated. This material tends to fade over time and does not hold up to normal quality standards in the jewelry industry. The best turquoises are usually found in arid regions.
The cobweb matrix already mentioned can increase the value of a stone. This shape is very popular in the US Southwest and Asia , but it is disliked in the Middle East , where pure, blue turquoise is preferred. Uniform coloring is desirable, and craftsmanship also counts in processed pieces. This includes the polishing quality and the symmetry of the stone. Calibrated stones - those that meet the standards of the jewelry industry - are accordingly preferred. The price of turquoise, like coral, mostly depends on the size in millimeters and not on the weight.
Turquoise is treated in a wide variety of ways, with some methods being more permanent and radical than others. There is disagreement among experts as to whether or not some of these methods are acceptable, but generally “light” waxing or oiling to improve the color and shine of the turquoise is “allowed”. The prerequisite for this is that the quality of the source mineral is very high and only very little wax or oil is absorbed, i.e. the stone does not need regular care to maintain its beauty. In general, however, untreated turquoise is always more expensive than treated or artificial.
As a phosphate mineral, turquoise is very sensitive to acidic or basic solutions. Sweat affects the stone just as much as perfumes, skin oils and other cosmetics or cleaning agents such as soap . Turquoise jewelry should therefore be removed when washing your hands and cleaning, as its color can turn into an unsightly, brownish green over time. Turquoise is just as sensitive to heat. A temperature of around 250 ° C, which can easily be reached when soldering or polishing , makes the stone take on a green color. If the stone is exposed to direct sunlight for a long time, it will lose color and / or crystal water (it will dehydrate ). Therefore, when wearing turquoise jewelry, make sure that cosmetics, sunscreen and hairspray are applied before putting on the jewelry. It should not be worn on the beach or for sunbathing. To prevent deposits, it can be gently cleaned with a soft cloth after wearing. A separate box is suitable for storage in order to avoid being scratched by other gemstones.
Manipulations and imitations
The Egyptians seem to have been the first to be able to produce artificial turquoise using the glazed clay product Egyptian faience . Later, glass and enamel were also used and in modern times more sophisticated ceramic products, porcelain , plastic and other reconstructed, pressed, glued and fired raw materials for the production of artificial turquoise were created. The latter consist of various copper and aluminum components.
Examples would be "Viennese turquoise" (Viennese turquoise), made from precipitated aluminum orthophosphate and colored with copper oleates, as well as "Neolith" (Reese turquoise), a mixture of bayerite and copper phosphates . Both products differ significantly from the original in terms of physical and chemical properties. Another turquoise imitation is known as "neo turquoise" and is made using gibbsite and copper phosphate.
In 1972 Pierre Gilson succeeded in producing something like synthetic turquoise. Due to the glue used, however, it differs in its chemical composition, so it is better to call it imitation than synthetic . Gilson's turquoise comes in a uniform color and with the black “cobweb” matrix (spiderweb turquoise ), which is not unlike the turquoise from Nevada.
Due to the widespread use of artificially treated, imitated or synthetically manufactured material, the popularity of turquoise has recently been impaired. Even experts often cannot distinguish such pieces from real natural stones.
Due to the similar color or appearance, which is helped with artificial coloring, turquoise can be confused with many minerals or mineral adhesions. Amazonite , chrysocolla , Faustite , hemimorphite , lazulite , serpentine , smithsonite , utalite , variscite , as well as an intergrowth of variscite, chalcedony and quartz known as amatrix (American matrix) are similar in color . Amatrix is easy to distinguish from turquoise due to its Mohs hardness of 7.
The most widespread imitation bases are howlite (hardness 3 to 3.5) and magnesite (hardness 4), both of which are white in their original form, colored blue but look extremely similar to the coveted matrix turquoise. Dyed chalcedony, jasper , and marble are not as common or look that real. Another frequently encountered imitation is the odontolite or "bone turquoise ", a fossil bone that is colored using the mineral Vivianite . Odontolite used to be mined on a large scale in the south of France specifically for the production of turquoise , but is now very much out of fashion.
All these fakes can be unmasked by gemologists by means of numerous tests, whereby these rely heavily on a thorough examination of the surface structure under the microscope , during which the stone must not be destroyed. Natural turquoise has a light blue background with white spots or dots. The artificially produced stones, however, are fundamentally different in terms of color (usually a solid dark blue) and surface texture (granular, similar to sugar). Glass and plastics are more translucent, sometimes with bubbles or small streaks under the surface. Spots between the area near the grain boundary are visible in colored imitations.
In some cases, however, the stone is destroyed. Dilute hydrochloric acid dissolves the carbonates odontolite and magnesite with the formation of bubbles and howlite turns green. In plastic products, heating the stone produces a pungent odor. Original and forgery can also be distinguished from one another in terms of their density , refractive index, light absorption (see absorption spectrum of turquoise) and other physical and optical properties. Artificial turquoise is so common today that it has long been superior to natural turquoise in terms of quantity. It can now be assumed that over 90% of the turquoise sold are treated, reconstructed or imitated in some way. Even in “authentic” Indian and Tibetan jewelry, one often only finds artificial or, in the best case, heavily treated turquoise.
Methods of rectification
Natural turquoise is seldom hard and durable enough to be processed into jewelry in an untreated form . Turquoise is therefore enhanced in different ways before and after sanding . The first methods of touching up were light waxing and oiling, which can increase the shine and intensify the color. These methods are now accepted as a tradition, as the starting material is usually of a higher quality anyway. Modern treatment methods such as the pressure impregnation of otherwise unsaleable calcareous American turquoise using epoxy resin , polystyrene and water glass ( alkali silicates ), however, encounter resistance. They are seen as too radical an encroachment on nature. Plastic and alkali silicate are technically superior to oil and wax in terms of durability and stability, as they can also be used on turquoise, which would be too brittle for the older methods. Turquoise treated in this way is called "reconstructed" or "stabilized" turquoise. The epoxy resin method was invented by Colbaugh Processing of Arizona in the 1950s. The majority of American turquoise is treated this way today, although the process itself is very expensive and takes several months. Without impregnation , most American mines would not be competitive.
Stones that are oiled and treated with wax tend to "sweat" when exposed to little heat or when exposed to excessive sunlight. Their surface will turn white or cloudy over time. The use of Berlin blue and other colors - often in connection with adhesive treatments - to intensify, standardize or completely change the color is not only viewed as fraudulent by purists . In addition, some color additives fade over time or stain the carrier.
A coloring is also applied to enhance the dark streaks of turquoise. The most radical method is certainly the “reconstruction”, whereby splinters that are too small to be processed otherwise are simply glued together and a larger stone is formed. Many of these replicas, if not all, usually no longer contain any natural components or have been filled with foreign minerals (see imitations). Another treatment method - of which no detailed information is known - is the so-called Zachery process, which was named after its inventor, the electrical engineer and turquoise trader James E. Zachery. Allegedly only mediocre stones are used here. The turquoise is harder after the treatment and has a nicer color and a better shine.
Since high-quality turquoise is usually only found in thin gaps, it is combined with harder material for reinforcement. The result is a duplicate that is used in certain jewelry designs. Sometimes the turquoise bedrock is also used as a base. Duplicates, like all of the previously mentioned methods, are legal as long as the buyer is advised of this prior to purchase.
As is so often the case with gemstones, this is precisely not the case, which is why gemologists often have to examine the suspicious stones. By heating it is relatively easy to determine whether the stone has been treated with oil, wax or plastic.
see also: Main article gemstone: manipulations and imitations
Shamanism and esotericism
Among the so-called " Native Americans " such as the Aztecs , Hohokam , Moche , Navajo and Zuñi , the turquoise played an outstanding, mythical and spiritual role and was considered a powerful protective and healing stone that, for example, saved travelers from harm and when used correctly Shamans could cure diseases. The Aztec rain god ( Tlaloc ) was tempered by gifts of turquoise and Quetzalcoatl was responsible for the manual skills of the ancient Mexican stone cutting artists ("lapidaries").
In China turquoise was less important than jade , but just like in Tibet it was considered a stone for luck and protection that could give joy, health and a long life. In Egypt and Persia, too, it was said to have strong protective properties; the deceased received it in the form of jewelry and amulets as grave goods .
In modern esotericism , turquoise is used as a healing stone for various ailments, mostly in combination with others. It is said to help with inflammatory diseases, for example, if it is worn on the body (necklace or bracelet) or the water in which a turquoise lay is drunk. There is no scientific evidence for this.
Various astrologers assign turquoise to Mercury (according to Raphael, 1987), Venus (according to Ahlborn, 1996), Jupiter (according to Richardson and Huett, 1989) or Uranus (according to Uyldert, 1983). As a zodiac stone it is assigned to Aquarius and as a monthly stone to December.
See also
literature
Monographs:
- Abdelhadi Ahmed, Eduard Gübelin, Werner Lieber, Guanghua Liu, Christoph Meister, Stefan Weiß: Turquoise: The gemstone with the color of the sky . In: Christian Weise (ed.): ExtraLapis . tape 16 . Christian Weise Verlag, 1999, ISBN 3-921656-48-6 , ISSN 0945-8492 .
- Turquoise , In: John W. Anthony, Richard A. Bideaux, Kenneth W. Bladh, Monte C. Nichols (Eds.): Handbook of Mineralogy, Mineralogical Society of America , 2001 ( PDF 68 kB )
- Uwe Kolitsch, G. Giester: The crystal structure of faustite and its copper analogue turquoise . (PDF; 537 kB) In: Mineralogical Magazine , 64, 2000, pp. 905-913.
- H. Cid-Dresdner: Determination and refinement of the crystal structure of turquois, CuAl6 (PO4) 4 (OH) 8 * 4H2O . (PDF; 1.4 MB) In: Zeitschrift fur Kristallographie , 121, 1965, pp. 87–113.
- JE Pogue: The turquoise: a study of its history, mineralogy, geology, ethnology, archeology, mythology, folklore, and technology . National Academy of Sciences, The Rio Grande Press, Glorieta NM 1915, ISBN 0-87380-056-7 .
In compendia:
- Walter Schumann: Precious stones and gemstones. All kinds and varieties. 1900 unique pieces . 16th revised edition. BLV Verlag, Munich 2014, ISBN 978-3-8354-1171-5 , pp. 186 .
- Petr Korbel, Milan Novák: Encyclopedia of Minerals . Dörfler Verlag GmbH, Eggolsheim 2002, ISBN 978-3-89555-076-8 , p. 182 .
- R. Webster: Gems: Their sources, descriptions and identification . 5th ed., Butterworth-Heinemann, Great Britain 2000, ISBN 0-7506-1674-1 , pp. 254-263.
- H. Schadt: Goldsmith's art: 5000 years of jewelry and hollowware . Arnoldsche Art Publisher, Stuttgart / New York 1996, ISBN 3-925369-54-6
- Cornelius S. Hurlbut, Cornelis Klein: Manual of Mineralogy , 20th ed. John Wiley and Sons, New York 1985, ISBN 0-471-80580-7
Web links
- Mineralienatlas: Turquoise and Mineralienatlas: Mineralienportrait / Turquoise (wiki)
- Mindat - Turquoise (English)
- Webmineral - Turquoise (English)
- EPI Institute for Gem Testing - Turquoise
- American Mineralogist Crystal Structure Database
Individual evidence
- ^ RJ King: Turquoise . In: Geology Today . No. 18 (3) . Geological Society of London , 2002, p. 110-114 , doi : 10.1046 / j.1365-2451.2002.00345.x .
- ↑ a b c d e f g Walter Schumann: Precious stones and gemstones. All species and varieties in the world. 1600 unique pieces . 13th revised and expanded edition. BLV Verlag, Munich 2002, ISBN 3-405-16332-3 , p. 186 .
- ↑ a b Mineralienatlas: Mineralienportrait / Turquoise
- ^ Waldemar Theodore Schaller : Crystallized turquoise from Virginia . In: American Journal of Science Volume 33, 1912, pp. 35-40
- ↑ a b Stefan Weiß: The large Lapis mineral directory. All minerals from A - Z and their properties . 6th completely revised and supplemented edition. Weise, Munich 2014, ISBN 978-3-921656-80-8 .
- ↑ Mindat - Rashleighite (English)
- ↑ Mindat - Turquoise
- ↑ Jürgen Ehlers (ed. And trans.): Abū'l-Qāsem Ferdausi: Rostam - The legends from the Šāhnāme . Philipp Reclam jun., Stuttgart 2002, p. 372
- ↑ a b c Bernhard Bruder: Decorated stones . Neue Erde Verlag, 2005, ISBN 3-89060-025-5 , p. 105-109 .
- ↑ a b Abdelhadi Ahmed, Eduard Gübelin, Werner Lieber, Guanghua Liu, Christoph Meister, Stefan Weiß: Turquoise: The gemstone with the color of the sky . In: Christian Weise (ed.): ExtraLapis . tape 16 . Christian Weise Verlag, 1999, ISBN 3-921656-48-6 , ISSN 0945-8492 , p. 24, 58, 60 .
- ^ Walter Schumann: Precious stones and gemstones. All species and varieties in the world. 1600 unique pieces . 13th revised and expanded edition. BLV Verlag, Munich 2002, ISBN 3-405-16332-3 , p. 284-286 .