State of matter: Difference between revisions
m Reverting possible vandalism by 59.94.241.117 to version by Faithlessthewonderboy. False positive? Report it. Thanks, User:ClueBot. (416037) (Bot) |
No edit summary |
||
| Line 101: | Line 101: | ||
[[eo:Fazo de materio]] |
[[eo:Fazo de materio]] |
||
[[es:Estado de agregación de la materia]] |
[[es:Estado de agregación de la materia]] |
||
[[fa:حالتهای ماده]] |
|||
[[fi:Faasi]] |
[[fi:Faasi]] |
||
[[fr:Phase (matière)]] |
[[fr:Phase (matière)]] |
||
Revision as of 16:36, 12 June 2008
A state of matter is a class of materials, usually solid, liquid, and gas. Ionized Plasma, Quark-gluon plasma, Bose-Einstein condensate and fermionic condensate are other less commonly known states of matter. A state of matter is also referred to as a physical state and often erroneously described as a phase. There is a classic general science description of each of the phases: A solid is a material that maintains its shape and its volume; a liquid maintains its volume but takes on the shape of its container; A gas takes on both the shape and volume of its container. Bose-Einstein condensate and fermionic condensate are currently achievable at near absolute zero temperatures in laboratory settings. Both kinds of plasma are achieved at very high temperatures, and behave as gases. Quark-gluon plasmas exist at such high temperatures and pressures that they are unlikely to exist outside of laboratory conditions since shortly after the Big Bang.
Solids
Materials that are solids have a stable, definite shape, and a definite volume. In a solid, the particles are packed closely together, they cannot move freely, they can only vibrate. The movement energy and temperature are very low.
Liquids
Materials that are liquids do not have a definite shape. The shape of a liquid is determined by the container in which it is contained. The volume is definite. In a liquid, the particles are farther apart, and they can slide past each other easily. The movement energy and temperature, in comparison to a solid, are higher.
Gases
Materials that are gases have an indefinite, unstable shape. The volume is determined by the container that is closely sealed. In a gas, the particles are far apart from each other, and they can move around quickly. The movement energy and temperature are the higher than those of both solids and liquids.
Plasmas
Plasmas are known as the fourth state of matter. They are "hotter" than gas. A plasma occurs when the temperature is between 1000 degrees C and 1,000,000,000 degrees C. Some examples of plasma are flames, lightning, aurora (northern lights), neon lights, and stars, including our own sun.
Ions are chemical species that contain unequal number of electrons and protons, and therefore possess an electrical charge. As plasmas are heated, electrons begin to leave the charged species, resulting in the presence of free electrons, which are not bound to an atom or molecule. The free electric charges make the plasma electrically conductive so that it responds strongly to electromagnetic fields. At very high temperatures, such as those present in stars, it is assumed that essentially all electrons are "free," and that a very high energy plasma is essentially bare nuclei swimming in a sea of electrons. The most common state of matter in the universe is believed to be plasma.
Bose-Einstein condensate
In 1924, Albert Einstein and Satyendra Bose predicted the "Bose-Einstein condensate," the fifth state of matter. An example is helium-4 close to absolute zero in the superfluid state, in which it will attempt to 'climb' out of its container.[1]
In the gas phase, the Bose-Einstein condensate remained an unverified theoretical prediction for many years. Finally in 1995, Wolfgang Ketterle and his team of graduate students produced such a condensate experimentally. A Bose-Einstein condensate is "colder" than a solid. It occurs when atoms have very similar (or the same) quantum levels. Temperatures close to absolute zero (-273 °C) will exhibit the Bose-Einstein condensate.
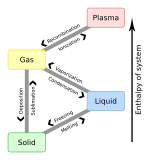
Changes in states of matter

a solid → a liquid = melting (heat energy added) e.g. ice melts to water
a liquid → a gas = evaporation (heat energy added) e.g. water to water vapour
a solid → a gas = sublimation (heat energy added) e.g. dried ice (frozen CO2) to carbon dioxide
a gas → a liquid = condensation (heat energy removed) e.g. cloud to rain
a liquid → a solid = solidification (heat energy removed) e.g. water to ice
a gas → a solid = deposition (heat energy removed) e.g. water vapour to frost
Other examples of states of matter
Under extremely high pressure, ordinary matter undergoes a transition to a series of exotic states of matter collectively known as degenerate matter. These are of great interest to astrophysics, because these high-pressure conditions are believed to exist inside stars that have used up their nuclear fusion "fuel", such as white dwarves and neutron stars.
When in a normal solid state, the atoms of matter align themselves in a grid pattern, so that the spin of any electron is the opposite of the spin of all electrons touching it. But in a string-net liquid, atoms are arranged in some pattern which would require some electrons to have neighbors with the same spin. This gives rise to some curious properties, as well as supporting some unusual proposals about the fundamental conditions of the universe, itself.
One of the metastable states of strongly non ideal plasma is Rydberg matter which forms upon condensation of excited atoms. These atoms can also turn into ions and electrons if they reach a certain temperature.
See also
References
External links
- 2005-06-22, MIT News: MIT physicists create new form of matter Citat: "... They have become the first to create a new type of matter, a gas of atoms that shows high-temperature superfluidity."
- 2003-10-10, Science Daily: Metallic Phase For Bosons Implies New State Of Matter
- 2004-01-15, ScienceDaily: Probable Discovery Of A New, Supersolid, Phase Of Matter Citat: "...We apparently have observed, for the first time, a solid material with the characteristics of a superfluid...but because all its particles are in the identical quantum state, it remains a solid even though its component particles are continually flowing..."
- 2004-01-29, ScienceDaily: NIST/University Of Colorado Scientists Create New Form Of Matter: A Fermionic Condensate