Immunoreceptor tyrosine-based activation motif: Difference between revisions
No edit summary |
No edit summary |
||
| Line 6: | Line 6: | ||
== Function == |
== Function == |
||
[[Image:TCRComplex.png|thumb|The '''T-cell receptor complex''' with TCR-α and TCR-β chains, [[CD3 receptor|CD3]] and ζ-chain accessory molecules. ITAMs are represented in blue on the tails of the CD3 subunits.]] |
[[Image:TCRComplex.png|thumb|The '''T-cell receptor complex''' with TCR-α and TCR-β chains, [[CD3 receptor|CD3]] and ζ-chain accessory molecules. ITAMs are represented in blue on the tails of the CD3 subunits.]] |
||
ITAMs are important for signal transduction, mainly in immune cells. They are found in the cytoplasmic tails of non-catalytic tyrosine-[[Phosphorylation|phosphorylated]] receptors <ref name="Dushek2012">{{cite journal | vauthors = Dushek O, Goyette J, van der Merwe PA | title = Non-catalytic tyrosine-phosphorylated receptors | journal = Immunological Reviews | volume = 250 | issue = 1 | pages = 258–76 | date = November 2012 | pmid = 23046135 | pmc = | doi = 10.1111/imr.12008 }}</ref> such as the [[CD3 receptor|CD3]] and [[ζ-chain]]s of the [[T cell receptor]] complex<ref name=basic/>, the [[CD79]]-alpha and -beta chains of the B cell receptor complex, and certain [[Fc receptor]]s.<ref name="Dushek2012"/> The tyrosine residues within these motifs become phosphorylated by [[Src family kinase]]s following interaction of the receptor molecules with their [[ligand]]s. Phosphorylated ITAMs serve as docking sites for other proteins containing a [[SH2 domain]], usually two domains in tandem, inducing a signaling cascade mediated by Syk family kinases (which are the primary proteins that bind to phosphorylated ITAMs), namely either [[Tyrosine-protein kinase SYK|Syk]] or [[ZAP70|ZAP-70]], resulting in the activation of |
ITAMs are important for signal transduction, mainly in immune cells. They are found in the cytoplasmic tails of non-catalytic tyrosine-[[Phosphorylation|phosphorylated]] receptors <ref name="Dushek2012">{{cite journal | vauthors = Dushek O, Goyette J, van der Merwe PA | title = Non-catalytic tyrosine-phosphorylated receptors | journal = Immunological Reviews | volume = 250 | issue = 1 | pages = 258–76 | date = November 2012 | pmid = 23046135 | pmc = | doi = 10.1111/imr.12008 }}</ref> such as the [[CD3 receptor|CD3]] and [[ζ-chain]]s of the [[T cell receptor]] complex<ref name=basic/>, the [[CD79]]-alpha and -beta chains of the B cell receptor complex, and certain [[Fc receptor]]s.<ref name="Dushek2012"/> The tyrosine residues within these motifs become phosphorylated by [[Src family kinase]]s following interaction of the receptor molecules with their [[ligand]]s. Phosphorylated ITAMs serve as docking sites for other proteins containing a [[SH2 domain]], usually two domains in tandem, inducing a signaling cascade mediated by Syk family kinases (which are the primary proteins that bind to phosphorylated ITAMs), namely either [[Tyrosine-protein kinase SYK|Syk]] or [[ZAP70|ZAP-70]], resulting mostly in the activation of given cell. Paradoxically, in some cases, ITAMs and ITAM-like motifs do not have an activating effect, but rather an inhibitory one.<ref>{{Cite journal|last=Pasquier|first=Benoit|last2=Launay|first2=Pierre|last3=Kanamaru|first3=Yutaka|last4=Moura|first4=Ivan C.|last5=Pfirsch|first5=Séverine|last6=Ruffié|first6=Claude|last7=Hénin|first7=Dominique|last8=Benhamou|first8=Marc|last9=Pretolani|first9=Marina|last10=Blank|first10=Ulrich|last11=Monteiro|first11=Renato C.|date=2005-01|title=Identification of FcalphaRI as an inhibitory receptor that controls inflammation: dual role of FcRgamma ITAM|url=https://pubmed.ncbi.nlm.nih.gov/15664157/|journal=Immunity|volume=22|issue=1|pages=31–42|doi=10.1016/j.immuni.2004.11.017|issn=1074-7613|pmid=15664157}}</ref><ref>{{Cite journal|last=O’Neill|first=Shannon K.|last2=Getahun|first2=Andrew|last3=Gauld|first3=Stephen B.|last4=Merrell|first4=Kevin T.|last5=Tamir|first5=Idan|last6=Smith|first6=Mia J.|last7=Dal Porto|first7=Joseph M.|last8=Li|first8=Quan-Zhen|last9=Cambier|first9=John C.|date=2011-11-23|title=Monophosphorylation of CD79a and b ITAM motifs initiates a SHIP-1 phosphatase-mediated inhibitory signaling cascade required for B cell anergy|url=https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3232011/|journal=Immunity|volume=35|issue=5|pages=746–756|doi=10.1016/j.immuni.2011.10.011|issn=1074-7613|pmc=3232011|pmid=22078222}}</ref><ref>{{Cite journal|last=Pfirsch-Maisonnas|first=Séverine|last2=Aloulou|first2=Meryem|last3=Xu|first3=Ting|last4=Claver|first4=Julien|last5=Kanamaru|first5=Yutaka|last6=Tiwari|first6=Meetu|last7=Launay|first7=Pierre|last8=Monteiro|first8=Renato C.|last9=Blank|first9=Ulrich|date=2011-04-19|title=Inhibitory ITAM Signaling Traps Activating Receptors with the Phosphatase SHP-1 to Form Polarized “Inhibisome” Clusters|url=https://stke.sciencemag.org/content/4/169/ra24|journal=Science Signaling|language=en|volume=4|issue=169|pages=ra24–ra24|doi=10.1126/scisignal.2001309|issn=1945-0877|pmid=21505186}}</ref> Exact mechanisms of this phenomenon are as of yet not elucidated. |
||
Other non-catalytic tyrosine-phosphorylated receptors carry a conserved inhibitory motif ([[immunoreceptor tyrosine-based inhibitory motif|ITIM]]) that, when phosphorylated, results in the inhibition of the signaling pathway via recruitment of phosphatases, namely [[PTPN6|SHP-1]], [[PTPN11|SHP-2]] and [[INPP5D|SHIP1]]. This serves not only for inhibition and regulation of signalling pathways related to ITAM-based signalling, but also for termination of signalling.<ref>{{Cite journal|last=Long|first=Eric O.|date=2008-08|title=Negative signaling by inhibitory receptors: the NK cell paradigm|url=https://pubmed.ncbi.nlm.nih.gov/18759921/|journal=Immunological Reviews|volume=224|pages=70–84|doi=10.1111/j.1600-065X.2008.00660.x|issn=1600-065X|pmc=2587243|pmid=18759921}}</ref><ref>{{Cite journal|last=Kane|first=Barry A.|last2=Bryant|first2=Katherine J.|last3=McNeil|first3=H. Patrick|last4=Tedla|first4=Nicodemus T.|date=2014|title=Termination of Immune Activation: An Essential Component of Healthy Host Immune Responses|url=https://www.karger.com/Article/FullText/363449|journal=Journal of Innate Immunity|language=english|volume=6|issue=6|pages=727–738|doi=10.1159/000363449|issn=1662-811X|pmid=25033984}}</ref><ref>{{Cite journal|last=Ligeti|first=E.|last2=Csépányi-Kömi|first2=R.|last3=Hunyady|first3=L.|date=2012-04|title=Physiological mechanisms of signal termination in biological systems|url=https://pubmed.ncbi.nlm.nih.gov/22260256/|journal=Acta Physiologica (Oxford, England)|volume=204|issue=4|pages=469–478|doi=10.1111/j.1748-1716.2012.02414.x|issn=1748-1716|pmid=22260256}}</ref> |
Other non-catalytic tyrosine-phosphorylated receptors carry a conserved inhibitory motif ([[immunoreceptor tyrosine-based inhibitory motif|ITIM]]) that, when phosphorylated, results in the inhibition of the signaling pathway via recruitment of phosphatases, namely [[PTPN6|SHP-1]], [[PTPN11|SHP-2]] and [[INPP5D|SHIP1]]. This serves not only for inhibition and regulation of signalling pathways related to ITAM-based signalling, but also for termination of signalling.<ref>{{Cite journal|last=Long|first=Eric O.|date=2008-08|title=Negative signaling by inhibitory receptors: the NK cell paradigm|url=https://pubmed.ncbi.nlm.nih.gov/18759921/|journal=Immunological Reviews|volume=224|pages=70–84|doi=10.1111/j.1600-065X.2008.00660.x|issn=1600-065X|pmc=2587243|pmid=18759921}}</ref><ref>{{Cite journal|last=Kane|first=Barry A.|last2=Bryant|first2=Katherine J.|last3=McNeil|first3=H. Patrick|last4=Tedla|first4=Nicodemus T.|date=2014|title=Termination of Immune Activation: An Essential Component of Healthy Host Immune Responses|url=https://www.karger.com/Article/FullText/363449|journal=Journal of Innate Immunity|language=english|volume=6|issue=6|pages=727–738|doi=10.1159/000363449|issn=1662-811X|pmid=25033984}}</ref><ref>{{Cite journal|last=Ligeti|first=E.|last2=Csépányi-Kömi|first2=R.|last3=Hunyady|first3=L.|date=2012-04|title=Physiological mechanisms of signal termination in biological systems|url=https://pubmed.ncbi.nlm.nih.gov/22260256/|journal=Acta Physiologica (Oxford, England)|volume=204|issue=4|pages=469–478|doi=10.1111/j.1748-1716.2012.02414.x|issn=1748-1716|pmid=22260256}}</ref> |
||
Revision as of 19:07, 6 September 2020
An immunoreceptor tyrosine-based activation motif (ITAM) is a conserved sequence of four amino acids that is repeated twice in the cytoplasmic tails of Non-catalytic tyrosine-phosphorylated receptors, cell-surface proteins found mainly on immune cells.[1] Its major role is being an integral component for the initiation of a variety of signaling pathway and subsequently the activation of immune cells, although different functions have been described, for example an osteoclast maturation.[2][3]
Structure
The motif contains a tyrosine separated from a leucine or isoleucine by any two other amino acids, giving the signature YxxL/I.[1] Two of these signatures are typically separated by between 6 and 8 amino acids in the cytoplasmic tail of the molecule (YxxL/Ix(6-8)YxxL/I). However, it is worth noting that in various sources, this consensus sequence differs, mainly in the number of amino acids between individual signatures. Apart from ITAMs which have the structure described above, there is also a variety of proteins containing ITAM-like motifs, which have a very simillar structure and function (for example Dectin-1).[4][5][6]
Function
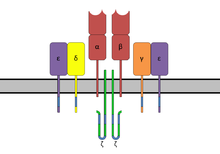
ITAMs are important for signal transduction, mainly in immune cells. They are found in the cytoplasmic tails of non-catalytic tyrosine-phosphorylated receptors [7] such as the CD3 and ζ-chains of the T cell receptor complex[1], the CD79-alpha and -beta chains of the B cell receptor complex, and certain Fc receptors.[7] The tyrosine residues within these motifs become phosphorylated by Src family kinases following interaction of the receptor molecules with their ligands. Phosphorylated ITAMs serve as docking sites for other proteins containing a SH2 domain, usually two domains in tandem, inducing a signaling cascade mediated by Syk family kinases (which are the primary proteins that bind to phosphorylated ITAMs), namely either Syk or ZAP-70, resulting mostly in the activation of given cell. Paradoxically, in some cases, ITAMs and ITAM-like motifs do not have an activating effect, but rather an inhibitory one.[8][9][10] Exact mechanisms of this phenomenon are as of yet not elucidated.
Other non-catalytic tyrosine-phosphorylated receptors carry a conserved inhibitory motif (ITIM) that, when phosphorylated, results in the inhibition of the signaling pathway via recruitment of phosphatases, namely SHP-1, SHP-2 and SHIP1. This serves not only for inhibition and regulation of signalling pathways related to ITAM-based signalling, but also for termination of signalling.[11][12][13]
Genetic Variations
Rare human genetic mutations are catalogued in the human genetic variation databases[14][15][16] which can reportedly result in creation or deletion of ITIM and ITAMs[17].
Examples
CD3γ, CD3δ, CD3ε, CTLA-4, PD-1, FcαRI, FcγRI, FcγRII, FcγRIII, Dectin-1, CLEC-1, CD28, CD72
References
- ^ a b c Abbas, Abul K; Lichtman, Andrew H. (2009), Basic Immunology: Functions and Disorders of the Immune System (3 ed.), Philadelphia, PA: Saunders, ISBN 978-1-4160-4688-2
{{citation}}: Unknown parameter|name-list-format=ignored (|name-list-style=suggested) (help) - ^ Humphrey, Mary Beth; Daws, Michael R.; Spusta, Steve C.; Niemi, Eréne C.; Torchia, James A.; Lanier, Lewis L.; Seaman, William E.; Nakamura, Mary C. (2006-02). "TREM2, a DAP12-associated receptor, regulates osteoclast differentiation and function". Journal of Bone and Mineral Research: The Official Journal of the American Society for Bone and Mineral Research. 21 (2): 237–245. doi:10.1359/JBMR.051016. ISSN 0884-0431. PMID 16418779.
{{cite journal}}: Check date values in:|date=(help) - ^ Paloneva, Juha; Mandelin, Jami; Kiialainen, Anna; Böhling, Tom; Prudlo, Johannes; Hakola, Panu; Haltia, Matti; Konttinen, Yrjö T.; Peltonen, Leena (2003-08-18). "DAP12/TREM2 Deficiency Results in Impaired Osteoclast Differentiation and Osteoporotic Features". Journal of Experimental Medicine. 198 (4): 669–675. doi:10.1084/jem.20030027. ISSN 0022-1007.
- ^ Rogers, Neil C.; Slack, Emma C.; Edwards, Alexander D.; Nolte, Martijn A.; Schulz, Oliver; Schweighoffer, Edina; Williams, David L.; Gordon, Siamon; Tybulewicz, Victor L.; Brown, Gordon D.; Reis e Sousa, Caetano (2005-04). "Syk-dependent cytokine induction by Dectin-1 reveals a novel pattern recognition pathway for C type lectins". Immunity. 22 (4): 507–517. doi:10.1016/j.immuni.2005.03.004. ISSN 1074-7613. PMID 15845454.
{{cite journal}}: Check date values in:|date=(help) - ^ Underhill, David M.; Rossnagle, Eddie; Lowell, Clifford A.; Simmons, Randi M. (2005-10-01). "Dectin-1 activates Syk tyrosine kinase in a dynamic subset of macrophages for reactive oxygen production". Blood. 106 (7): 2543–2550. doi:10.1182/blood-2005-03-1239. ISSN 0006-4971. PMC 1895265. PMID 15956283.
- ^ Suzuki-Inoue, Katsue; Fuller, Gemma L. J.; García, Angel; Eble, Johannes A.; Pöhlmann, Stefan; Inoue, Osamu; Gartner, T. Kent; Hughan, Sascha C.; Pearce, Andrew C.; Laing, Gavin D.; Theakston, R. David G. (2006-01-15). "A novel Syk-dependent mechanism of platelet activation by the C-type lectin receptor CLEC-2". Blood. 107 (2): 542–549. doi:10.1182/blood-2005-05-1994. ISSN 0006-4971. PMID 16174766.
- ^ a b Dushek O, Goyette J, van der Merwe PA (November 2012). "Non-catalytic tyrosine-phosphorylated receptors". Immunological Reviews. 250 (1): 258–76. doi:10.1111/imr.12008. PMID 23046135.
- ^ Pasquier, Benoit; Launay, Pierre; Kanamaru, Yutaka; Moura, Ivan C.; Pfirsch, Séverine; Ruffié, Claude; Hénin, Dominique; Benhamou, Marc; Pretolani, Marina; Blank, Ulrich; Monteiro, Renato C. (2005-01). "Identification of FcalphaRI as an inhibitory receptor that controls inflammation: dual role of FcRgamma ITAM". Immunity. 22 (1): 31–42. doi:10.1016/j.immuni.2004.11.017. ISSN 1074-7613. PMID 15664157.
{{cite journal}}: Check date values in:|date=(help) - ^ O’Neill, Shannon K.; Getahun, Andrew; Gauld, Stephen B.; Merrell, Kevin T.; Tamir, Idan; Smith, Mia J.; Dal Porto, Joseph M.; Li, Quan-Zhen; Cambier, John C. (2011-11-23). "Monophosphorylation of CD79a and b ITAM motifs initiates a SHIP-1 phosphatase-mediated inhibitory signaling cascade required for B cell anergy". Immunity. 35 (5): 746–756. doi:10.1016/j.immuni.2011.10.011. ISSN 1074-7613. PMC 3232011. PMID 22078222.
- ^ Pfirsch-Maisonnas, Séverine; Aloulou, Meryem; Xu, Ting; Claver, Julien; Kanamaru, Yutaka; Tiwari, Meetu; Launay, Pierre; Monteiro, Renato C.; Blank, Ulrich (2011-04-19). "Inhibitory ITAM Signaling Traps Activating Receptors with the Phosphatase SHP-1 to Form Polarized "Inhibisome" Clusters". Science Signaling. 4 (169): ra24–ra24. doi:10.1126/scisignal.2001309. ISSN 1945-0877. PMID 21505186.
- ^ Long, Eric O. (2008-08). "Negative signaling by inhibitory receptors: the NK cell paradigm". Immunological Reviews. 224: 70–84. doi:10.1111/j.1600-065X.2008.00660.x. ISSN 1600-065X. PMC 2587243. PMID 18759921.
{{cite journal}}: Check date values in:|date=(help) - ^ Kane, Barry A.; Bryant, Katherine J.; McNeil, H. Patrick; Tedla, Nicodemus T. (2014). "Termination of Immune Activation: An Essential Component of Healthy Host Immune Responses". Journal of Innate Immunity. 6 (6): 727–738. doi:10.1159/000363449. ISSN 1662-811X. PMID 25033984.
- ^ Ligeti, E.; Csépányi-Kömi, R.; Hunyady, L. (2012-04). "Physiological mechanisms of signal termination in biological systems". Acta Physiologica (Oxford, England). 204 (4): 469–478. doi:10.1111/j.1748-1716.2012.02414.x. ISSN 1748-1716. PMID 22260256.
{{cite journal}}: Check date values in:|date=(help) - ^ Auton A, Brooks LD, Durbin RM, Garrison EP, Kang HM, Korbel JO, et al. (October 2015). "A global reference for human genetic variation". Nature. 526 (7571): 68–74. Bibcode:2015Natur.526...68T. doi:10.1038/nature15393. PMC 4750478. PMID 26432245.
- ^ Sherry ST, Ward MH, Kholodov M, Baker J, Phan L, Smigielski EM, Sirotkin K (January 2001). "dbSNP: the NCBI database of genetic variation". Nucleic Acids Research. 29 (1): 308–11. doi:10.1093/nar/29.1.308. PMC 29783. PMID 11125122.
- ^ Cummings BB, Karczewski KJ, Kosmicki JA, Seaby EG, Watts NA, Singer-Berk M, et al. (May 2020). "Transcript expression-aware annotation improves rare variant interpretation". Nature. 581 (7809): 452–458. Bibcode:2020Natur.581..452C. doi:10.1038/s41586-020-2329-2. PMC 7334198. PMID 32461655.
- ^ Ulaganathan VK (May 2020). "TraPS-VarI: Identifying genetic variants altering phosphotyrosine based signalling motifs". Scientific Reports. 10 (1): 8453. Bibcode:2020NatSR..10.8453U. doi:10.1038/s41598-020-65146-2. PMC 7242328. PMID 32439998.
