Osteopetrosis
| Classification according to ICD-10 | |
|---|---|
| Q78.2 | Marble Bone Disease |
| ICD-10 online (WHO version 2019) | |
The osteopetrosis (including osteopetrosis , marble bone disease , Albers-Schonberg disease , Albers-Schonberg syndrome ) is in the majority of cases by a hereditary underactive bone-degrading cells ( osteoclasts causes). The undirected accumulation of bone tissue disrupts the microarchitecture of the bone and reduces its mechanical stability.
Since the osteoclasts are formed from precursor cells of the white blood cells in the course of hematopoiesis , the only therapy to date has been a bone marrow transplant , after which functional osteoclasts are formed and the excessive sclerotherapy is reduced, with a considerable risk of hypercalcaemia .
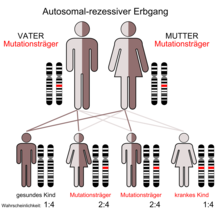
Classification and inheritance
According to their inheritance, the various forms of osteopetrosis are classified into two main groups:
- autosomal recessive forms (infantile malignant osteopetrosis, osteopetrosis with renal tubular acidosis )
- autosomal dominant forms (type 1 (ADOI), type 2 (ADOII, Albers-Schönberg disease))
Course of autosomal recessive osteopetrosis
The autosomal recessive osteopetrosis usually begins in the first two years of life, the form with renal tubular acidosis a little later than the infantile malignant form, which can lead to symptoms in the first few weeks after birth. However, there are also milder recessive clinical pictures that can overlap with the dominant osteopetrosis. If left untreated, the prognosis is usually poor. The only chance of a cure is currently in a bone marrow transplant because the defective osteoclasts from hematopoietic lineage derived.
Course of autosomal dominant osteopetrosis
Generalized osteosclerosis with a special emphasis on the base of the skull is typical for ADOI, while for ADOII, which corresponds to the disease originally described by Albers-Schönberg, the so-called "sandwich vertebrae" in the X-ray image are characteristic. Both dominant forms often become symptomatic during the growth spurt in adolescence, with ADOI not leading to increased bone fractures but to increased stability. Cosmetic problems can arise from an enlargement of the lower jaw. The ADOI differs from all other forms because it is caused by an overactive osteoblast . In contrast, ADOII leads to frequent bone fractures and, in individual cases, to complications similar to the malignant form (mainly damage to the cranial nerves). There is currently no causal therapy.
Genetic causes
- Autosomal recessive osteopetrosis:
Osteoclast-rich forms: TCIRG1 , CLCN7 , OSTM1 , SNX10
Forms low in osteoclasts: TNFSF11 (RANKL), TNFRSF11A (RANK)
- Autosomal recessive osteopetrosis with renal tubular acidosis: CAII
- Autosomal dominant osteopetrosis type 1: LRP5
- Autosomal dominant osteopetrosis type 2: CLCN7
Pathogenesis
Osteoblasts play a crucial role in regulating osteoclast activity. If there is a defect in CSF-1 synthesis, which is secreted by osteoblasts, osteoclasts cannot differentiate (the fusion of osteoclast mononuclear progenitor cells becomes impossible). Because there are no osteoclasts, the bone does not break down, which leads to osteopetrosis.
The transcription factor c-Fos must also be expressed in the osteoclastic progenitor cells so that further differentiation takes place. The responsible gene was switched off in mice, which led to osteoclast deficiency and consequently to osteopetrosis.
Osteoclasts are particularly rich in actin filaments and C-Src kinase in the so-called adhesive zone. The gene deactivation of the C-Src kinase also leads to osteopetrosis in mice, as it does not adhere to the bone matrix.
Osteoclasts also release lysosomal enzymes through exocytosis, including tartrate-resistant acid phosphatase (TRAP) and cathepsin K, which promote bone breakdown. The genetically caused cathepsin K deficiency leads to pycnodysostosis, an important differential diagnosis of osteopetrosis, characterized by skeletal abnormalities, short stature and increased bone mass.
In addition, osteoclasts have the chloride channel ClC-7 in their brush border (Ruffled Border) , which releases chloride into the lacuna between the osteoclast and the bone matrix. This is of central importance for bone resorption, as hydrochloric acid is formed in the lacuna. ClC-7 mutations lead to autosomal recessive or autosomal dominant osteopetrosis.
Carbonic anhydrase -II is a critical enzyme in osteoclasts that enables the formation of hydrogen ions and hydrogen carbonate from water and carbon dioxide. The hydrogen ions are then pumped into the lacuna, the hydrogen carbonate is exchanged for chloride in the smooth side of the cell (away from the bone matrix) with the participation of anion exchanger 2 (AE2). A genetic defect in carbonic anhydrase II leads to osteopetrosis.
clinic
Despite a significant increase in bone mass, fractures occur frequently , which are often difficult to heal. Enlargement of the liver and spleen (due to extramedullary blood formation ), reduced immune defense , seizures and damage to cranial nerves (especially blindness) can occur as further complications .
Diagnosis and differential diagnosis
The x-ray shows a considerably increased bone density and a narrowing of the medullary canal. Often clumped metaphyses of the long tubular bones with horizontal stripes. Thickening of the roof and base of the skull.
Are to be delimited
- Pycnodysostosis
- Melorheostosis
- Sclerostosis
- Engelmann syndrome (progressive diaphyseal dysplasia)
- Pyle syndrome
literature
- Kornak et al.: Molecular mechanisms of the regulation of bone density by osteoclasts. In: Ärzteblatt. Vol. 100, No. 19, pp. A1258-A1268.
- U. Kornak, S. Mundlos: Genetic disorders of the skeleton: a developmental approach . In: Am. J. Hum. Genet. tape 73 , no. 3 , September 2003, p. 447-474 , doi : 10.1086 / 377110 , PMID 12900795 , PMC 1180673 (free full text).
Individual evidence
- ↑ Rukshana Shroff, Ortraud Beringer, Kanchan Rao, Lorenz C. Hofbauer, Ansgar Schulz: Denosumab for Post-Transplantation Hyeprcalcemia on Osteopetrosis. In: New England Journal of Medicine. Volume 367, Issue 18, November 1, 2012, pp. 1766–1767.
- ↑ D. Drenkhahn (Ed.): Anatomie. Volume 1, 16th edition. Urban & Fischer, Munich 2003, pp. 133-149.
- ↑ F. Hefti: Pediatric Orthopedics in Practice . Springer 1998, ISBN 3-540-61480-X , p. 674.