Atherton-Todd reaction
The Atherton-Todd reaction is a name reaction in organic chemistry that goes back to the British chemists FR Atherton, HT Openshaw and AR Todd . They first described the reaction in 1945 as a method for converting dialkyl phosphites into dialkyl chlorophosphates. The dialkyl chlorophosphates formed are often too reactive to be isolated . For this reason, in the presence of alcohols or amines, the synthesis of phosphates or phosphoramidates can follow the Atherton-Todd reaction.
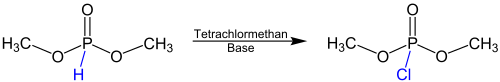
Overview reaction
The following reaction equation gives an overview for the Atherton-Todd reaction using the example of dimethyl phosphite as a reactant :
The reaction takes place with the addition of carbon tetrachloride and a base . This base is usually a primary , secondary or tertiary amine . Instead of the methyl groups , other alkyl groups or benzyl groups can also be present.
Reaction mechanism
A possible reaction mechanism for the Atherton-Todd reaction will be explained in accordance with the overview reaction using the example of the starting compound dimethyl phosphite:
First, a tertiary amine is used to split off a methyl group from the dimethyl phosphite . Intermediate stage 1 results from this reaction step .
This is followed by protonation of intermediate 1 by the starting compound dimethyl phosphite. In turn, this is deprotonated, so that intermediate stage 2a and intermediate stage 2b are formed. Then intermediate stage 1 is recovered from intermediate stage 2a .
Finally, intermediate 2b is chlorinated using carbon tetrachloride and dimethyl chlorophosphate 3 is formed .
Possible further course of the reaction
After the synthesis of the dimethyl chlorophosphate, a further reaction, for example with a primary amine (e.g. aniline ), is possible according to the following reaction equation:
Atomic economy
In addition to the dialkyl phosphite, the starting compound used in this reaction is tetrachloromethane in a stoichiometric amount and a base (amine). At the same time, the starting compound itself is used as a reagent , but not in a stoichiometric amount. Thus, only chloroform , which emerges from carbon tetrachloride after two reaction steps, is relevant as a waste product for assessing the atomic economy . It should also be borne in mind that the product of the reaction has a greater molar mass than the starting compound. The atom economy of this reaction can therefore be classified as relatively good.
See also
The Atherton-Todd reaction is related to the Appel reaction . Here, too, tetrachloromethane is used for chlorination .
Individual evidence
- ^ FR Atherton, HT Openshaw, AR Todd: 174. Studies on phosphorylation. Part II. The reaction of dialkyl phosphites with polyhalogen compounds in the presence of bases. A new method for the phosphorylation of amines . In: Journal of the Chemical Society (Resumed) . 1945, p. 660-663 , doi : 10.1039 / jr9450000660 .
- ↑ a b Zerong Wang: Comprehensive organic name reactions and reagents Volume 1 . John Wiley, Hoboken (NJ) 2009, ISBN 978-0-470-28662-3 , pp. 114-118 .
- ^ Stéphanie S. Le Corre, Mathieu Berchel, Hélène Couthon-Gourvès, Jean-Pierre Haelters, Paul-Alain Jaffrès: Atherton – Todd reaction: mechanism, scope and applications . In: Beilstein Journal of Organic Chemistry . tape 10 , no. 1 , 2014, p. 1166-1196 , doi : 10.3762 / bjoc.10.117 .