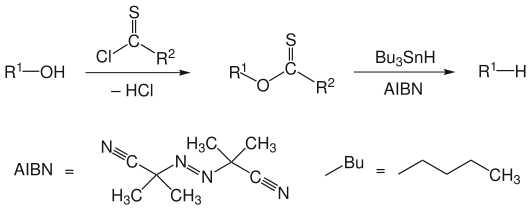
Barton-McCombie deoxygenation
The Barton-McCombie deoxygenation is a name reaction in organic chemistry . It is named after the British chemists Derek Harold Richard Barton and Stuart W. McCombie . The reaction describes a two-stage conversion in which the functional hydroxyl group of a compound is formally substituted by a hydrogen atom. In the first reaction step , the alcohol is converted to the thione ester , and in the second reaction step , radical deoxygenation with tributylstannane and a radical initiator such as AIBN takes place . Not all leaving groups are listed in the following scheme .
R 1 is an alkyl radical , R 2 can be H−, CH 3 -, CH 3 S−, CH 3 O−, Ph -, PhO− or an imidazolyl radical.
Sulfur-tin bonds are very stable. The formation of such a bond is the driving force behind the entire reaction.
mechanism
The reaction mechanism consists of a catalytic initiation of a radical and the subsequent propagation steps (chain reaction). The alcohol ( 1 ) is first converted into a more reactive thiocarboxy derivative, such as e.g. B. a thionic acid ester or a xanthate ( 2 ). Then a tributylstannyl radical ( 4 ) is generated by reacting tributylstannane ( 3 ) with azobis (isobutyronitrile) (AIBN) ( 8 ). The tributylstannyl radical separates the xanthate group from ( 2 ), thus forming a tributyltin xanthate ( 7 ) and leaving behind an alkyl radical ( 5 ). The alkyl radical, in turn, abstracts a hydrogen atom from a new tributylstannane molecule ( 3 ), thus providing the desired deoxygenated product ( 6 ) and generating a new radical species from ( 4 ), which in turn can enter into the subsequent reaction.
Variations
Alternative sources of hydrogen
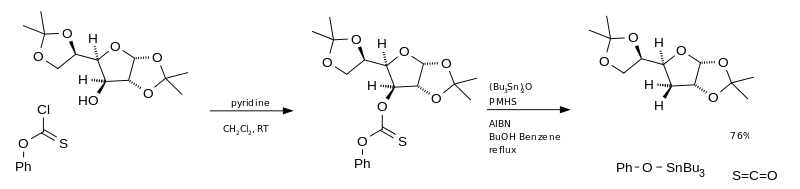
The main disadvantage of this reaction is the use of tributylstannane. This is very toxic, expensive and difficult to separate from the reaction mixture. An alternative is the use of bis (tributyltin) oxide (TBTO) as the radical source and polymethylhydrosiloxane (PMHS) as the hydrogen source. When using O-phenyl chlorothioformate as the starting material, carbonyl sulfide is produced in the end .
Atomic economy
Barton-McCombie deoxygenation delivers the desired product, but it must be taken into account that this reaction generates significant stoichiometric amounts of waste. Thus, although the reaction is effective, it has poor atomic economy . The residues of the radical initiator (here: isobutyronitrile) and the thio product must be disposed of. This makes Barton deoxygenation uneconomical as an industrial process, but it can be used for synthesis in the laboratory.
The Barton-McCombie deoxygenation should not be confused with the Barton reaction or the closely related Barton decarboxylation .
Web links
- Barton-McCombie deoxygenation in the Organic Chemistry Portal
- Barton-McCombie deoxygenation in the Organische-Chemie.ch portal
- Article on alkyl boranes
Individual evidence
- ↑ Barton, DHR ; McCombie, SW: A new method for the deoxygenation of secondary alcohols . In: J. Chem. Soc., Perkin Trans. 1 . 16, 1975, pp. 1574-1585. doi : 10.1039 / P19750001574 .
- ↑ Crich, D .; Quintero, L .: Radical chemistry associated with the thiocarbonyl group . In: Chem Rev.. . 89, 1989, pp. 1413-1432. doi : 10.1021 / cr00097a001 .
- ↑ Forbes, JE; Zard, SZ Tetrahedron Lett. 1989 , 30 , 4367.
- ↑ Jordi Tormo, Gregory C. Fu : Tributylstannane (Bu3SnH) -Catalyzed Barton − McCombie Deoxygenation of Alcohols: 3-Deoxy-1,2: 5,6-bis-O- (1-methylethylidene) -α-D-ribo- hexofuranose In: Organic Syntheses . 78, 2002, p. 239, doi : 10.15227 / orgsyn.078.0239 ( PDF ).