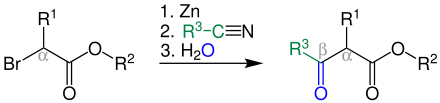Blaise reaction
The Blaise reaction is a name reaction from organic chemistry. It was published for the first time in 1901 by the French chemist Edmond Blaise (1872–1939). An α-bromo ester reacts with a nitrile via zinc initially to form a β-enamino ester and, after acidic hydrolysis, to a β-keto ester.
General
The Blaise reaction has been improved or modified in many ways since 1901. Nowadays it is known that processing with a concentrated solution of potassium carbonate (K 2 CO 3 ) produces mostly β-amino esters 1 during hydrolysis and β- keto esters 2 during subsequent acidic hydrolysis . The reaction mechanism is initially based on the Reformatsky reaction . Quite high yields of 78% can be achieved even at room temperature.
Proposed reaction mechanism
First, zinc inserts into the Br – C bond of 1 , in the manner of a Reformatzki reaction # mechanism , in the process the organic zinc compound 2 is formed . In the next step there is an electrophilic attack by the zinc on the triple bond of the nitrile. This loosens the C – Zn bond and creates a bond with the positive carbon atom of the nitrile; via intermediate 3 , 4 is formed . A β- enamino ester 5 is then formed in aqueous solution . By adding an acid (e.g. HCl) the hydrolysis of 6 takes place with the formation of the oxonium ion 7 . A 1,3 proton shift creates 8 . By splitting off an ammonium ion , the end product is the β-ketoester 9 :
literature
- Zerong Wang: Comprehensic Organic Name Reactions and Reagents. Volume 1, Wiley, 2009, pp. 424-428, ISBN 978-0-471-70450-8 .
- Bradford P. Mundy, Michael G. Ellerd, Frank G. Favaloro Jr .: Name Reactions and reagents in Organic Syntheses. 2nd edition, Wiley-Interscience, 2005, p. 98, ISBN 0-471-22854-0 .
Individual evidence
- ↑ EE Blaise: In: Compt. Rend. Volume 132, 1901, p. 478.
- ↑ (a) SM Hannick and Y. Kishi: In: Journal of Organic Chemistry . Volume 48, 1983, p. 3833. (b) https://www.organische-chemie.ch/OC/Namen/blaise-reaktion.htm
- ^ J. Pospisil and IE Marko: In: Journal of the American Chemical Society . Volume 129, 2007, p. 3516.
- ^ Zerong Wang: Comprehensic Organic Name Reactions and Reagents. Volume 1, Wiley, 2009, p. 425, ISBN 978-0-471-70450-8 .