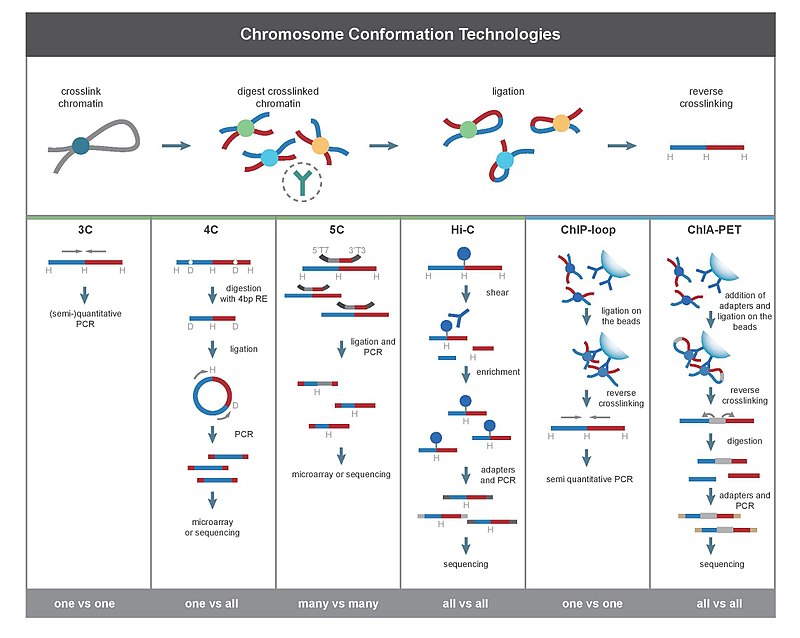
Chromosome conformation capture
Chromosome conformation capture (Eng. " Conformation capture of chromosomes ", often abbreviated to 3C technologies or 3C-based methods) are a number of molecular biological methods with which the spatial organization of chromatin in a cell is analyzed. These methods quantify the number of interactions between genomic loci that are in close proximity in three-dimensional space but can be separated by many nucleotides in the linear genome. Such interactions can result from biological functions such as B. promoter - enhancer interactions, or result from a random loop formation in which an undirected physical movement of the chromatin leads to a collision of the loci. The frequency of these interactions can be analyzed directly, or they can be converted into distances and used to reconstruct 3D structures. This technology has further supported the genetic and epigenetic study of chromosomes in both model organisms and humans.
The main difference between 3C-based methods is their scope. For example, if PCR is used to detect interactions in a 3C experiment, then the interactions between two specific fragments are quantified. In contrast, the Hi-C method quantifies the interactions between all possible pairs of fragments simultaneously.
Experimental methods
All 3C methods start with a similar set of steps performed on a cell sample.
First, the cell genomes are cross-linked with formaldehyde . This introduces bonds that "freeze" the interactions between genomic locations. Treatment of cells with 1-3% formaldehyde for 10-30 minutes at room temperature is the most common. However, standardization is necessary to prevent protein-rich DNA crosslinking, since this can negatively affect the efficiency of the restriction digest in the subsequent step. The genome is then broken up into fragments with a restriction endonuclease . For this purpose, restriction enzymes are used which cut recognition sequences with a length of 6 bp such as EcoR1 or HindIII . These cut the genome approximately every 4000bp. This results in around 1 million fragments in the human genome. The size of the restriction fragments determines the resolution of the interaction mapping. A restriction enzyme of 4bp can also be used for a more precise mapping of the interactions.
The next step is the random linkage ( ligation ) of the fragments. The end pieces of the fragments can be made to join by complementary base pairing. This happens at low DNA concentrations in the presence of T4 DNA ligase. The link between crosslinked, interacting fragments is preferred here over the link between non-crosslinked fragments. Subsequently, interacting loci are quantified by amplifying ligated compounds using PCR methods.
Original methods
3C (one-vs-one)
The Chromosome Confirmation Capture (3C) experiment quantifies the interactions between a single pair of genomic loci. For example, 3C can be used to test the possible interaction between promoter and enhancer. Ligated fragments are detected by means of PCR with known primers .
4C (one-vs-all)
Chromosome conformation capture-on-chip (4C) captures interactions between a locus and all other genomic loci. It is a second ligation step to generate self-circular DNA fragments that are used to perform the inverse PCR. The inverse PCR makes it possible to use a known sequence to amplify the unknown sequence attached to it. In contrast to the 3C and 5C methods, the 4C technique does not require any prior knowledge of both interacting chromosomal regions. The results obtained with 4C are highly reproducible for most of the interactions seen between the nearby areas. About a million interactions can be analyzed on a single microarray .
5C (many-vs-many)
Chromosome conformation capture carbon copy (5C) recognizes interactions between all restriction fragments within a certain area, whereby the size of this area is typically no larger than a megabase. However, 5C has relatively poor coverage. The 5C technique overcomes some problems in the intramolecular ligation step and is useful for constructing complex interactions of the loci under study. This approach is unsuitable for performing genome-wide complex interactions because it would require the use of millions of 5C primers.
Hi-C (all-vs-all)
Hi-C uses high throughput sequencing to find the nucleotide sequence of fragments. The original protocol used shotgun sequencing , which retrieves a short sequence from each end of each ligated fragment. Therefore, the two sequences obtained for a particular ligated fragment should represent two different restriction fragments that were ligated together in the random ligation step. The pair of sequences are individually aligned with the genome and thus determine the fragments involved in this ligation event. Therefore, all possible pair-wise interactions between the fragments are tested.
Researchers are trying to investigate the possibility of Hi-C detection through a study that focuses on screening for primary brain tumors. Prior to such tumor studies, Hi-C mainly focused on analyzing cell lines.
Methods based on sequence acquisition
A number of methods use oligonucleotide detection to enrich 3C and Hi-C libraries for specific loci of interest. These methods include Capture-C, NG Capture-C, Capture-3C, and Capture Hi-C. These methods are able to produce a higher resolution and sensitivity than 4C-based methods.
Methods for single cells
With Single-Cell Hi-C, the interactions that occur in individual cells can also be examined.
Methods based on immunoprecipitation
ChIP loop
ChIP-loop combines 3C with ChIP-seq to detect interactions between two loci under investigation that are mediated by a protein. ChIP-loop can be useful to identify long-range cis-interactions and trans-interactions that are mediated by proteins, since frequent DNA collisions do not occur.
Genome-wide methods
ChIA-PET combines Hi-C with ChIP-Seq in order to detect all interactions that are mediated by a protein of interest. HiChIP was developed to enable an analysis similar to ChIA-PET with less starting material.
Biological effects
3C methods have led to a number of biological insights, including the discovery of new structural features of chromosomes, the cataloging of chromatin loops, and a better understanding of the mechanisms of transcriptional regulation (the disruption of which can lead to disease).
3C methods have shown the importance of the physical proximity of regulatory elements to the genes that regulate them. For example, in tissues that express globin genes, the control region β-globin locus forms a loop with these genes. This loop is not found in tissues where the gene is not expressed. This technology has further supported the genetic and epigenetic study of chromosomes in both model organisms and humans.
These methods have resulted in a large-scale organization of the genome into Topologically Associating Domains (TADs) that correlate with epigenetic markers. Some TADs are transcriptionally active while others are suppressed. Many TADs have been found in D. melanogaster , mouse and human. In addition, the proteins CTCF and play cohesin an important role in the determination of TADs and enhancerpromoter interactions. The result shows that the alignment of the CTCF binding motifs in an enhancer-promoter loop should face each other for the enhancer to find its correct target.
Human diseases
There are several diseases that are caused by improper promoter-enhancer interactions.
- Beta thalassemia is a specific type of blood disorder caused by a deletion of the LCR enhancer.
- Holoprosencephaly is a headache condition caused by a mutation in the SBE2 enhancer element. This in turn weakens the production of the SHH gene.
- Lung adenocarcinoma can be caused by duplicating the enhancer for the MYC gene.
- The adult T-cell leukemia is caused by the introduction of a new enhancer.
Data analysis
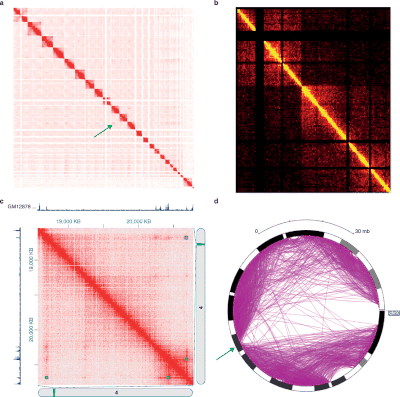
The various experiments with the 3C method generate data with very different structures and statistical properties. Therefore there are specific analysis packages for each experiment type.
Hi-C data are often used to analyze genome-wide chromatin organization, such as topologically associating domains (TADs), linearly contiguous areas of the genome that are close together in three-dimensional space in the nucleus. Several algorithms have been developed to identify TADs from Hi-C data.
Hi-C and its subsequent analyzes are evolving. Fit-Hi-C is a procedure based on a discrete binning approach with modifications of the added interaction distance (initial spline fitting, alias Spline-1) and the refinement of the null model (Spline-2). The result of Fit-Hi-C is a list of pairwise intra-chromosomal interactions with their p-values and q-values.
A major disruptive factor in 3C technologies are the frequent non-specific interactions between genomic loci that occur due to random polymer behavior. An interaction between two loci has to be confirmed as specific by statistical significance tests.
Hi-C contact card normalization
There are two main ways of normalizing raw Hi-C contact heatmaps . The first way is to assume uniform visibility, i.e. H. there is an equal chance for each chromosomal position to have an interaction. Therefore, the true signal of a Hi-C contact card should be a symmetrical matrix (the symmetrical matrix has constant row and column sums). An example of algorithms that assume the same visibility is the Sinkhorn-Knopp algorithm, which scales the original Hi-C contact card into a balanced matrix.
The other variant is to assume that each chromosomal position is assigned a bias . The value in the contact card at each coordinate is the real signal multiplied by the bias associated with the two contact positions. An example of algorithms aimed at solving this model of distortion is iterative correction, which gradually removes row and column distortion from the raw Hi-C contact card. A number of software tools are available for analyzing Hi-C data.
DNA motif analysis
DNA motifs are specific short DNA sequences, often 8-20 nucleotides long, that are statistically over-represented in a set of sequences with a common biological function. At present (2019), regulatory motives for long-range chromatin interactions have not yet been comprehensively investigated. Several studies have focused on examining the effects of DNA motifs on the interaction between promoter and enhancer.
Bailey et al. have identified that the ZNF143 motif in the promoter regions offers specificity for the sequences for promoter-enhancer interactions. The mutation of the ZNF143 motif decreased the frequency of promoter-enhancer interactions, suggesting that ZNF143 is a novel factor in chromatin looping.
Analysis of cancer genomes
The 3C-based techniques can provide insights into the chromosomal rearrangements in the cancer genome. In addition, they can show changes in the spatial proximity of regulatory elements and their target genes, which enable a deeper understanding of the structural and functional basis of the genome.
Individual evidence
- ^ De Wit E, de Laat W: A decade of 3C technologies: insights into nuclear organization . In: Genes & Development . 26, No. 1, January 2012, pp. 11-24. doi : 10.1101 / gad.179804.111 . PMID 22215806 . PMC 3258961 (free full text).
- ↑ a b c d Hakim O, Misteli T: SnapShot: Chromosome confirmation capture . In: Cell . 148, No. 5, March 2012, pp. 1068.e1-2. doi : 10.1016 / j.cell.2012.02.019 . PMID 22385969 . PMC 6374129 (free full text).
- ↑ a b c Ay F, Bailey TL, Noble WS: Statistical confidence estimation for Hi-C data reveals regulatory chromatin contacts . In: Genome Research . 24, No. 6, June 2014, pp. 999-1011. doi : 10.1101 / gr.160374.113 . PMID 24501021 . PMC 4032863 (free full text).
- ↑ Rao SS, Huntley MH, Durand NC, Stamenova EK, Bochkov ID, Robinson JT, Sanborn AL, Machol I, Omer AD, Lander ES, Aiden EL: A 3D map of the human genome at kilobase resolution reveals principles of chromatin looping . In: Cell . 159, No. 7, December 2014, pp. 1665-80. doi : 10.1016 / j.cell.2014.11.021 . PMID 25497547 . PMC 5635824 (free full text).
- ↑ Varoquaux N, Ay F, Noble WS, Vert JP: A statistical approach for inferring the 3D structure of the genome . In: Bioinformatics . 30, No. 12, June 2014, pp. I26-33. doi : 10.1093 / bioinformatics / btu268 . PMID 24931992 . PMC 4229903 (free full text).
- ↑ Alexey Gavrilov, Elvira Eivazova, Iryna Pirozhkova, Marc Lipinski, Sergey Razin: Chromosome Conformation Capture (from 3C to 5C) and Its ChIP-Based Modification . In: Chromatin Immunoprecipitation Assays . tape 567 . Humana Press, Totowa, NJ 2009, ISBN 978-1-60327-413-5 , pp. 171–188 , doi : 10.1007 / 978-1-60327-414-2_12 ( springer.com [accessed July 21, 2019]).
- ↑ Naumova N, Smith EM, Zhan Y, Dekker J: Analysis of long-range chromatin interactions using Chromosome Conformation Capture . In: Methods . 58, No. 3, November 2012, pp. 192-203. doi : 10.1016 / j.ymeth.2012.07.022 . PMID 22903059 . PMC 3874837 (free full text).
- ↑ Belton JM, Dekker J: Chromosome Conformation Capture (3C) in Budding Yeast . In: Cold Spring Harbor Protocols . 2015, No. 6, June 2015, pp. 580–6. doi : 10.1101 / pdb.prot085175 . PMID 26034304 .
- ↑ Gavrilov AA, AK Golov, Razin SV: Actual ligation frequencies in the chromosome conformation capture procedure . In: PLOS ONE . 8, No. 3, March 26, 2013, p. E60403. bibcode : 2013PLoSO ... 860403G . doi : 10.1371 / journal.pone.0060403 . PMID 23555968 . PMC 3608588 (free full text).
- ↑ Naumova N, Smith EM, Zhan Y, Dekker J: Analysis of long-range chromatin interactions using Chromosome Conformation Capture . In: Methods . 58, No. 3, November 2012, pp. 192-203. doi : 10.1016 / j.ymeth.2012.07.022 . PMID 22903059 . PMC 3874837 (free full text).
- ↑ Gavrilov AA, AK Golov, Razin SV: Actual ligation frequencies in the chromosome conformation capture procedure . In: PLOS ONE . 8, No. 3, March 26, 2013, p. E60403. bibcode : 2013PLoSO ... 860403G . doi : 10.1371 / journal.pone.0060403 . PMID 23555968 . PMC 3608588 (free full text).
- ^ Nuclear organization of active and inactive chromatin domains uncovered by chromosome conformation capture-on-chip (4C) . In: Nature Genetics . 38, No. 11, November 2006, pp. 1348-54. doi : 10.1038 / ng1896 . PMID 17033623 .
- ↑ Lieberman-Aiden E, van Berkum NL, Williams L, Imakaev M, Ragoczy T, Telling A, Amit I, Lajoie BR, Sabo PJ, Dorschner MO, Sandstrom R, Bernstein B, Bender MA, Groudine M, Gnirke A, Stamatoyannopoulos J, Mirny LA, Lander ES, Dekker J: Comprehensive mapping of long-range interactions reveals folding principles of the human genome . In: Science . 326, No. 5950, October 2009, pp. 289-93. bibcode : 2009Sci ... 326..289L . doi : 10.1126 / science.1181369 . PMID 19815776 . PMC 2858594 (free full text).
- ↑ Harewood L, Kishore K, Eldridge MD, Wingett S, Pearson D, Schoenfelder S, Collins VP, Fraser P: Hi-C as a tool for precise detection and characterization of chromosomal rearrangements and copy number variation in human tumors . In: Genome Biology . 18, No. 1, June 2017, p. 125. doi : 10.1186 / s13059-017-1253-8 . PMID 28655341 . PMC 5488307 (free full text).
- ↑ Burton JN, Adey A, Patwardhan RP, Qiu R, Kitzman JO, Shendure J: Chromosome-scale scaffolding of de novo genome assemblies based on chromatin interactions . In: Nature Biotechnology . 31, No. 12, December 2013, pp. 1119-25. doi : 10.1038 / nbt.2727 . PMID 24185095 . PMC 4117202 (free full text).
- ↑ Schmitt AD, Hu M, Ren B: Genome-wide mapping and analysis of chromosome architecture . In: Nature Reviews. Molecular Cell Biology . 17, No. 12, December 2016, pp. 743-755. doi : 10.1038 / nrm.2016.104 . PMID 27580841 . PMC 5763923 (free full text).
- ↑ Hughes JR, Roberts N, McGowan S, Hay D, Giannoulatou E, Lynch M, De Gobbi M, Taylor S, Gibbons R, Higgs DR: Analysis of hundreds of cis-regulatory landscapes at high resolution in a single, high-throughput experiment . In: Nature Genetics . 46, No. 2, February 2014, pp. 205-12. doi : 10.1038 / ng.2871 . PMID 24413732 .
- ↑ Davies JO, Telenius JM, McGowan SJ, Roberts NA, Taylor S, Higgs DR, Hughes JR: Multiplexed analysis of chromosome conformation at vastly improved sensitivity . In: Nature Methods . 13, No. 1, January 2016, pp. 74-80. doi : 10.1038 / nmeth.3664 . PMID 26595209 . PMC 4724891 (free full text).
- ↑ Hughes JR, Roberts N, McGowan S, Hay D, Giannoulatou E, Lynch M, De Gobbi M, Taylor S, Gibbons R, Higgs DR: Analysis of hundreds of cis-regulatory landscapes at high resolution in a single, high-throughput experiment . In: Nature Genetics . 46, No. 2, February 2014, pp. 205-12. doi : 10.1038 / ng.2871 . PMID 24413732 .
- ↑ Jäger R, Migliorini G, Henrion M, Kandaswamy R, Speedy HE, Heindl A, Whiffin N, Carnicer MJ, Broome L, Dryden N, Nagano T, Schoenfelder S, Enge M, Yuan Y, Taipale J, Fraser P, Fletcher O, Houlston RS: Capture Hi-C identifies the chromatin interactome of colorectal cancer risk loci . In: Nature Communications . 6, February 2015, p. 6178. bibcode : 2015NatCo ... 6.6178J . doi : 10.1038 / ncomms7178 . PMID 25695508 . PMC 4346635 (free full text).
- ↑ Single-cell Hi-C reveals cell-to-cell variability in chromosome structure . In: Nature . 502, No. 7469, October 2013, pp. 59-64. bibcode : 2013Natur.502 ... 59N . doi : 10.1038 / nature12593 . PMID 24067610 . PMC 3869051 (free full text).
- ↑ Single-cell epigenomics: techniques and emerging applications . In: Nature Reviews. Genetics . 16, No. 12, December 2015, pp. 716–26. doi : 10.1038 / nrg3980 . PMID 26460349 .
- ↑ Horike S, Cai S, Miyano M, Cheng JF, Kohwi-Shigematsu T: Loss of silent-chromatin looping and impaired imprinting of DLX5 in Rett syndrome . In: Nature Genetics . 37, No. 1, January 2005, pp. 31-40. doi : 10.1038 / ng1491 . PMID 15608638 .
- ^ The second decade of 3C technologies: detailed insights into nuclear organization . In: Genes & Development . 30, No. 12, June 2016, pp. 1357-82. doi : 10.1101 / gad.281964.116 . PMID 27340173 . PMC 4926860 (free full text).
- ↑ Tolhuis B, Palstra RJ, Splinter E, Grosveld F, de Laat W: Looping and interaction between hypersensitive sites in the active beta-globin locus . In: Molecular Cell . 10, No. 6, December 2002, pp. 1453-65. doi : 10.1016 / S1097-2765 (02) 00781-5 . PMID 12504019 .
- ^ Cavalli G, Misteli T: Functional implications of genome topology . In: Nature Structural & Molecular Biology . 20, No. 3, March 2013, pp. 290-9. doi : 10.1038 / nsmb.2474 . PMID 23463314 . PMC 6320674 (free full text).
- ↑ Dekker J, Marti-Renom MA, Mirny LA: Exploring the three-dimensional organization of genomes: interpreting chromatin interaction data . In: Nature Reviews. Genetics . 14, No. 6, June 2013, pp. 390-403. doi : 10.1038 / nrg3454 . PMID 23657480 . PMC 3874835 (free full text).
- ↑ Guo Y, Xu Q, Canzio D, Shou J, Li J, Gorkin DU, Jung I, Wu H, Zhai Y, Tang Y, Lu Y, Wu Y, Jia Z, Li W, Zhang MQ, Ren B, Krainer AR, Maniatis T, Wu Q: CRISPR Inversion of CTCF Sites Alters Genome Topology and Enhancer / Promoter Function . In: Cell . 162, No. 4, August 2015, pp. 900-10. doi : 10.1016 / j.cell.2015.07.038 . PMID 26276636 . PMC 4642453 (free full text).
- ^ Regulation of disease-associated gene expression in the 3D genome . In: Nature Reviews. Molecular Cell Biology . 17, No. 12, December 2016, pp. 771–782. doi : 10.1038 / nrm.2016.138 . PMID 27826147 .
- ↑ Characterization of deletions which affect the expression of fetal globin genes in man . In: Nature . 279, No. 5714, June 1979, pp. 598-603. bibcode : 1979Natur.279..598F . doi : 10.1038 / 279598a0 . PMID 450109 .
- ↑ gamma-beta-thalassemia studies showing that deletion of the gamma- and delta-genes influences beta-globin gene expression in man . In: Nature . 283, No. 5748, February 1980, pp. 637-42. doi : 10.1038 / 283637a0 . PMID 6153459 .
- ↑ A functional screen for sonic hedgehog regulatory elements across a 1 Mb interval identifies long-range ventral forebrain enhancers . In: Development . 133, No. 4, February 2006, pp. 761-72. doi : 10.1242 / dev.02239 . PMID 16407397 .
- ↑ Identification of focally amplified lineage-specific super-enhancers in human epithelial cancers . In: Nature Genetics . 48, No. 2, February 2016, pp. 176–82. doi : 10.1038 / ng.3470 . PMID 26656844 .
- ↑ Oncogene regulation. An oncogenic super-enhancer formed through somatic mutation of a noncoding intergenic element . In: Science . 346, No. 6215, December 2014, pp. 1373-7. doi : 10.1126 / science.1259037 . PMID 25394790 . PMC 4720521 (free full text).
- ↑ Lajoie BR, van Berkum NL, Sanyal A, Dekker J: My5C: web tools for chromosome conformation capture studies . In: Nature Methods . 6, No. 10, October 2009, pp. 690-1. doi : 10.1038 / nmeth1009-690 . PMID 19789528 . PMC 2859197 (free full text).
- ↑ Deng X, Ma W, Ramani V, Hill A, Yang F, Ay F, Berletch JB, Blau CA, Shendure J, Duan Z, Noble WS, Disteche CM: Bipartite structure of the inactive mouse X chromosome . In: Genome Biology . 16, No. 1, August 2015, p. 152. doi : 10.1186 / s13059-015-0728-8 . PMID 26248554 . PMC 4539712 (free full text).
- ↑ Rao SS, Huntley MH, Durand NC, Stamenova EK, Bochkov ID, Robinson JT, Sanborn AL, Machol I, Omer AD, Lander ES, Aiden EL: A 3D map of the human genome at kilobase resolution reveals principles of chromatin looping . In: Cell . 159, No. 7, December 2014, pp. 1665-80. doi : 10.1016 / j.cell.2014.11.021 . PMID 25497547 . PMC 5635824 (free full text).
- ^ Zhou X, Lowdon RF, Li D, Lawson HA, Madden PA, Costello JF, Wang T: Exploring long-range genome interactions using the WashU Epigenome Browser . In: Nature Methods . 10, No. 5, May 2013, pp. 375-6. doi : 10.1038 / nmeth.2440 . PMID 23629413 . PMC 3820286 (free full text).
- ^ Yardımcı GG, Noble WS: Software tools for visualizing Hi-C data . In: Genome Biology . 18, No. 1, February 2017, p. 26. doi : 10.1186 / s13059-017-1161-y . PMID 28159004 . PMC 5290626 (free full text).
- ^ Genome-wide mapping and analysis of chromosome architecture . In: Nature Reviews. Molecular Cell Biology . 17, No. 12, December 2016, pp. 743-755. doi : 10.1038 / nrm.2016.104 . PMID 27580841 . PMC 5763923 (free full text).
- ^ Cavalli G, Misteli T: Functional implications of genome topology . In: Nature Structural & Molecular Biology . 20, No. 3, March 2013, pp. 290-9. doi : 10.1038 / nsmb.2474 . PMID 23463314 . PMC 6320674 (free full text).
- ↑ Rao SS, Huntley MH, Durand NC, Stamenova EK, Bochkov ID, Robinson JT, Sanborn AL, Machol I, Omer AD, Lander ES, Aiden EL: A 3D map of the human genome at kilobase resolution reveals principles of chromatin looping . In: Cell . 159, No. 7, December 2014, pp. 1665-80. doi : 10.1016 / j.cell.2014.11.021 . PMID 25497547 . PMC 5635824 (free full text).
- ↑ Rao SS, Huntley MH, Durand NC, Stamenova EK, Bochkov ID, Robinson JT, Sanborn AL, Machol I, Omer AD, Lander ES, Aiden EL: A 3D map of the human genome at kilobase resolution reveals principles of chromatin looping . In: Cell . 159, No. 7, December 2014, pp. 1665-80. doi : 10.1016 / j.cell.2014.11.021 . PMID 25497547 . PMC 5635824 (free full text).
- ^ Yardımcı GG, Noble WS: Software tools for visualizing Hi-C data . In: Genome Biology . 18, No. 1, February 2017, p. 26. doi : 10.1186 / s13059-017-1161-y . PMID 28159004 . PMC 5290626 (free full text).
- ^ Iterative correction of Hi-C data reveals hallmarks of chromosome organization . In: Nature Methods . 9, No. 10, October 2012, pp. 999-1003. doi : 10.1038 / nmeth.2148 . PMID 22941365 . PMC 3816492 (free full text).
- ↑ Zambelli F, Pesole G, Pavesi G: Motif discovery and transcription factor binding sites before and after the next-generation sequencing era . In: Briefings in Bioinformatics . 14, No. 2, March 2013, pp. 225-37. doi : 10.1093 / bib / bbs016 . PMID 22517426 . PMC 3603212 (free full text).
- ↑ Bailey, SD, Zhang, X., Desai, K., Aid, M., Corradin, O., Cowper-Sal·lari, R., ... Lupien, M. (2015). ZNF143 provides sequence specificity to secure chromatin interactions at gene promoters. Nature Communications, 2, 6186. Retrieved from http://dx.doi.org/10.1038/ncomms7186
- ↑ Harewood L, Kishore K, Eldridge MD, Wingett S, Pearson D, Schoenfelder S, Collins VP, Fraser P: Hi-C as a tool for precise detection and characterization of chromosomal rearrangements and copy number variation in human tumors . In: Genome Biology . 18, No. 1, June 2017, p. 125. doi : 10.1186 / s13059-017-1253-8 . PMID 28655341 . PMC 5488307 (free full text).
- ↑ Taberlay PC, Achinger-Kawecka J, Lun AT, Buske FA, Sabir K, Gould CM, Zotenko E, Bert SA, Giles KA, Bauer DC, Smyth GK, Stirzaker C, O'Donoghue SI, Clark SJ: Three-dimensional disorganization of the cancer genome occurs coincident with long-range genetic and epigenetic alterations . In: Genome Research . 26, No. 6, June 2016, pp. 719–31. doi : 10.1101 / gr.201517.115 . PMID 27053337 . PMC 4889976 (free full text).