Criegee rearrangement
The Criegee rearrangement , named after the German chemist Rudolf Criegee (1902–1975), is a name reaction from the field of organic chemistry and was first mentioned in 1945.
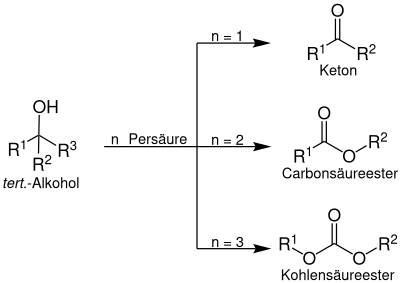
The Criegee rearrangement is a method of rearrangement of the peroxyester of a tertiary alcohol into ketones , esters and carbonates . The reaction takes place using a peracid . The Criegee rearrangement should not be confused with the Baeyer-Villiger oxidation , in which ketones are converted into esters by means of peracids.
Overview reaction
A tertiary alcohol is converted by means of peracid to a peroxyester, which can be converted to a ketone, a carboxylic acid ester or a carbonic acid ester by multiple insertion of oxygen:
Reaction mechanism
The reaction mechanism presented below is described in the literature:
First oxygen insertion
In the first step , the peroxyester 3 is formed from a tertiary alcohol 1 and a peracid 2 , with elimination of water .
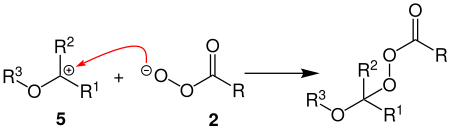
A strong polarization of the OO bond leads to heterolytic cleavage and intermediate stages 4 and 5 arise. The next step is the first insertion of oxygen. In the process, water is initially attached to the carbocation 5 . Proton rearrangement then leads to the splitting off of a primary alcohol and the carboxylate ion 4 splitting off a carboxylic acid . The product of the reaction is a ketone 6 .
Second oxygen insertion
During the second oxygen insertion, a peroxyester is initially formed again from the carbocation 5 with the peracid 2 .
Carbocation 7 is formed by renewed heterolytic cleavage of the OO bond of the peroxyester . As with the first oxygen insertion, a primary alcohol and a carboxylic acid are subsequently split off, a carboxylic acid ester 8 now being formed.
Third oxygen insertion
The third oxygen insertion proceeds according to the same scheme, with the peroxyester being formed from carbocation 7 .
The product of the third oxygen insertion is a carbonic acid ester 9 .
Individual evidence
- ↑ Rudolf Criegee: A new way into the cyclodecane series In: Ber. German Chem. Ges. 77, 1945, pp. 722-726, doi: 10.1002 / cber.19450770912 .
- ^ A b Zerong Wang: Comprehensive Organic Name Reactions and Reagents , Wiley, 2009, ISBN 978-0-471-70450-8 , pp. 770-774.
- ↑ R. Criegee: The rearrangement of the decalin peroxide esters as a result of cationic oxygen In: Justus Liebigs Ann. Chem. 560, 1948, pp. 127-135, doi: 10.1002 / jlac.19485600106 .