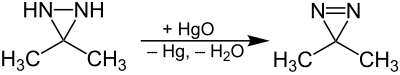Diazirine
| Diazirine |
|---|
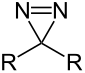 Diazirine (general formula) |
 3 H -diazirine |
 3,3-dimethyl-diazirine |
Diazirines are heterocyclic organic chemical substances that contain a three-membered ring consisting of two nitrogen atoms and one carbon atom, with the two nitrogen atoms forming an azo group (-N = N-). The diazirines belong to the larger group of three-ring compounds with two heteroatoms in the ring.
synthesis
Diazirines can be prepared from a corresponding amidine by the Graham reaction . Alternatively, the oxidation of a diaziridine also produces a diazirine:
Silver oxide (Ag 2 O) can also be used as an oxidizing agent .
properties
Diazirines break down into radical carbenes and elemental nitrogen after exposure to ultraviolet light . Pure diazirines can explode with the elimination of nitrogen:
Diazirines are used in biochemistry for photoaffinity labeling .
literature
- Michael TH Liu (Ed.): Chemistry of Diazirines , 1987. ISBN 978-0-8493-5047-4 .
- SM Korneev: Valence Isomerization between Diazo Compounds and Diazirines . In: Eur. J. Org. Chem. (2011), Volume 31, pp. 6153-6175, doi : 10.1002 / ejoc.201100224 .
Web links
Individual evidence
- ^ A b Siegfried Hauptmann : Organic Chemistry , 2nd revised edition, VEB Deutscher Verlag für Grundstoffindindustrie, Leipzig 1985, ISBN 3-342-00280-8 , p. 561.
- ^ Theophil Eicher , Siegfried Hauptmann, Andreas Speicher: The Chemistry of Heterocycles, Wiley-VCH, 2012, ISBN 978-3-527-32747-8 , p. 40.
- ^ M. Suchanek, A. Radzikowska, C. Thiele: Photo-leucine and photo-methionine allow identification of protein-protein interactions in living cells. In: Nature methods . Volume 2, Number 4, April 2005, pp. 261-267, doi : 10.1038 / nmeth752 , PMID 15782218 .
- ↑ AL MacKinnon, JL Garrison, RS Hegde, J. Taunton: Photo-leucine incorporation reveals the target of a cyclodepsipeptide inhibitor of cotranslational translocation. In: Journal of the American Chemical Society . Volume 129, number 47, November 2007, pp. 14560-14561, doi : 10.1021 / ja076250y , PMID 17983236 , PMC 2574519 (free full text).
- ↑ GA Korshunova, NV Sumbatian, AN Topin, MT Mchedlidze: [Photoactivated reagents based on aryl (trifluoromethyl) diazirines: synthesis and use for studying nucleic acid-protein interactions]. In : olekuliarnaia biologiia . Volume 34, Number 6, 2000 Nov-Dec, pp. 966-983, PMID 11186014 .