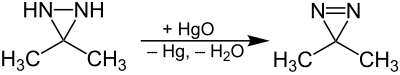Diaziridines
| Diaziridines |
|---|
 Diaziridine |
Diaziridines are heterocyclic organic chemical substances that contain a three-membered ring consisting of two nitrogen atoms and one carbon atom, with the two nitrogen atoms forming an azine group (–NN–). The diaziridines belong to the larger group of three-ring compounds with two heteroatoms in the ring.
properties
Some diaziridines are neurotrophic .
synthesis
A carbonyl reacts with ammonia or a primary amine and an amination reagent such as hydroxylamine- O- sulfonic acid (HOSA) under basic conditions. The aminal is then closed .
Reactivity
The oxidation of 3,3-dimethyl-diaziridine leads to 3,3-dimethyl-diazirine:
Unsubstituted diaziridines can also be oxidized with I 2 / NEt 3 to form the more stable diazirines . Diaziridines react with electrophilic groups such as ketenes or isocyanates with ring enlargement.
Individual evidence
- ↑ Synthesis of monocyclic diaziridines and their fused derivatives; NN Makhova, VY Petukhova, VV Kuznetsov, Arkivoc , 2008 (i), 128–152 ( PDF ).
- ^ A b Siegfried Hauptmann : Organic Chemistry , 2. Revised edition, VEB Deutscher Verlag für Grundstoffindindustrie, Leipzig, 1985, ISBN 3-342-00280-8 , p. 561.