Oil diffusion pump
The oil diffusion pump is a high vacuum pump that works on the principle of a jet pump . It has no moving parts and is therefore very reliable and easy to maintain. It is a further development of the diffusion pump introduced by Wolfgang Gaede in 1916 with mercury as a propellant.
Working method
- Main article: jet pump
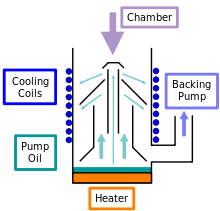
The pump consists of a cylindrical water- or air-cooled pump body. The bottom of the pump body is heated (usually electrically). Above is the boiling chamber with the propellant. The nozzle system through which the heated propellant flows at high pressure is located in the boiler room. Three to four cone-shaped steam jets emerge (one pump stage each), which condense on the cold walls.
Gas molecules that get into the steam jet are entrained and thus conveyed down into an area of higher pressure. The fore-vacuum nozzle is located there as the outlet from the diffusion pump. When the propellant is heated, the molecules exit again and are pumped out by the backing pump (e.g. rotary vane pump ).
Oil diffusion pumps require a fore-vacuum pressure of approx. 0.1 mbar ; they reach final pressures of up to approx. 10 −9 mbar, depending on the vapor pressure of the propellant.
In the area of the steam jet of the high vacuum stage, many propellant molecules emerge and from there reach the recipient . By using a cold trap , over 90% of these molecules can be trapped. Since contamination with pump oil significantly affects most vacuum processes, oil diffusion pumps are often combined with cold traps.
Propellant
High molecular weight substances are used as blowing agents. These are, for example, mineral oils that have been freed from their constituents with high vapor pressure under high vacuum, silicones and certain esters and ethers. Mineral oil-based oils have vapor pressures between 5 · 10 −7 and 2 · 10 −8 mbar at room temperature and good oxidation resistance. If there are higher demands on vapor pressure (ultimate vacuum) or resistance to oxidation, silicone oils, certain esters or pentaphenyl ethers (vapor pressure: 10 −10 mbar) are used.
In the past, only mercury was used as a propellant. Apart from the fact that mercury vapor is poisonous, mercury has a relatively high vapor pressure (approx. 10 −3 mbar) at cooling water temperature . Just to achieve a high vacuum, a cold trap has to be connected between the pump and the vacuum container in order to reduce the vapor pressure to sufficiently low values. Mercury diffusion pumps are only used in special cases, e.g. B. if mercury is already present in the container to be evacuated and therefore no cold trap has to be interposed.
Oil backflow
Nozzle hat vapor barriers ( cold cap ) and / or shell vapor barriers ( baffle ) prevent oil from flowing back into the recipient. Vapor barriers consist of cooled baffles that prevent oil molecules from flying through. To increase the temperature difference, liquid nitrogen (<77 K ) is also used for cooling .
disadvantage
Significant disadvantages of this type of pump are long heating and cooling times. In order to avoid this in batch operation (when containers are repeatedly opened and closed), the recipient is pre-evacuated with a bypass line before the oil diffusion pump is switched on.
Applications
- High vacuum pump for the high vacuum range (10 −3 to 10 −9 mbar, with delivery rates of up to 10 −6 mbar)
literature
Karl Jousten (Hrsg.): Wutz manual vacuum technology . 10th edition. Vieweg + Teubner, Wiesbaden 2010, ISBN 978-3-8348-0695-6 .