Acyl glycerols
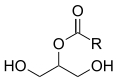
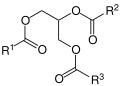
Acylglycerines (previously known as glycerides, glycerides or neutral fats) are organic chemical compounds of the trihydric alcohol glycerine and a maximum of three organic or inorganic acids that are linked by an ester bond . Depending on the ester bonds formed, a distinction is made between monoacyl, diacyl and triacylglycerols (mono-, di- and triglycerides).
Structure and division
|
||||||||||||||||||||||||||||||||||||||||||
| Note: –C (O) –R = fatty acids (acyl residues) |
All naturally occurring acylglycerols have the sn configuration , which defines the stereospecific spatial arrangement of the glycerol substituents.
Furthermore, the acylglycerols which are solid at room temperature are called fats , the liquid ones as oils .
Occurrence
Mono- and diacylglycerols have amphiphilic properties and can therefore be components of biomembranes or act as emulsifiers (e.g. when lipids pass from the intestine into the blood ). You can also carry a sugar residue on a remaining free hydroxyl group of the glycerine. These molecules are part of the cell envelope of bacteria , the thylakoid membrane of chloroplasts and the myelin sheath of nerve cells .

The most diverse group are the triacylglycerols , in which the glycerol is esterified with three fatty acids. Their structure is diverse, as there are different fatty acids and thus a large number of possible combinations. All are non-polar and therefore lipophilic. In the body they belong to storage and construction fat.
use
The mono- and diglycerides of fatty acids are used as additives ( E 471 ) in the food industry (R = long-chain alkyl or alkenyl residues of saturated or unsaturated fatty acids):
Individual evidence
- ↑ Gregory Cevc: Phospholipids Handbook. CRC Press, 1993, ISBN 9780824790509 , p. 2.