Elongation (translation)
| Parent |
| Translation |
| Gene Ontology |
|---|
| QuickGO |
The elongation during the translational treated the process of extension of the amino acid chain during the biosynthesis of the proteins ; it takes place at the location of the ribosome's recognition and binding . A single elongation step contains three steps: binding of the loaded tRNA , formation of the peptide bond and preparation for the next elongation step . This is repeated until a terminating codon is reached.
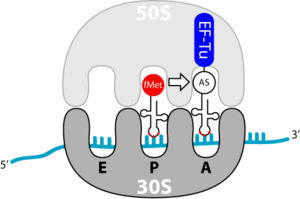
Binding of the aminoacyl tRNA in the A position
The elongation begins with the binding of a loaded tRNA in the A-site. This process is based on the transport through the elongation factor Tu ( EF-Tu ). This is again a G protein. EF-Tu has two important tasks:
- Protection of the ester bond between 3'-OH of the tRNA and the transported amino acid
- self-releasing when the correct tRNA has bound to the codon sequence of the A-site of the ribosome.
The discharge is due to the hydrolysis of the bound GTP. This can only take place with a correct pairing between ribosome and tRNA, i.e. when the correct tRNA is in the ribosome. An incorrect tRNA cannot dissociate from the EF-Tu, which guarantees the incorporation of the correct amino acid.
It is also interesting to note that EF-Tu can recognize any tRNA, except for the initiator tRNA, fmet-tRNA. This ensures that methionine is not incorporated into internal AUG codons, not fMet.
The formation of the peptide bond
This is a spontaneous step, due to the spatial conditions of the amino acids to each other and the greater bond strength of the peptide bond compared to the ester bond (between tRNA and AS). The 28S rRNA (!) Has the catalytic activity in this reaction. So the ribosome is a ribozyme. The reaction breaks the bond between the formyl methionine and the initiator tRNA. The initiator tRNA is thus discharged and a dipeptide is formed on the second tRNA. The peptide remains in the P site of the 50S UE (subunit) of the ribosome, but the 30S UE with mRNA and the two tRNAs are still in their starting position.
The reason for the formylation of the methionine on the amino group can now be seen. The dipeptide, which is formed after the first round of elongation, has the possibility of ring closure. The nitrogen of the methionine would have the possibility of a nucleophilic attack on the carboxyl carbon atom of the second amino acid. The dipeptide would detach from the tRNA and thus the elongation would terminate.
Restoring the initial situation
The translocation (movement) of the discharged initiator and the second tRNA is carried out by the elongation factor-G (EF-G). This has a molecular similarity to the EF-Tu-tRNA complex. It is believed that the EF-G, similar to the EF-Tu, binds to the 50S UE. The lower part of the EF-G resembles a tRNA, so an interaction with the 30S UE is obvious. Since EF-G is again a G protein, it is subject to a conformational change during GTP hydrolysis, i.e. it changes its structure. As a result, the tRNA-like part migrates towards the interior of the ribosome and "pushes" the two tRNAs one position further. The initiator tRNA is now at the E site, the second tRNA at the P site. With the dissociation of EF-G, the initial state is reached again. A new tRNA can bind to the A site and promote the growth of the peptide chain.
literature
- Kapp LD, Lorsch JR: The molecular mechanics of eukaryotic translation . In: Annu. Rev. Biochem. . 73, 2004, pp. 657-704. doi : 10.1146 / annurev.biochem.73.030403.080419 . PMID 15189156 .
Web links
- M. Anand, BA Balar, S. Gross, PA Ortiz, S. Ozturk, YR Pittman, R. Ulloque, TG Kinzy: Eukaryotic Translation Elongation. In: Reactome - a curated knowledgebase of biological pathways. 13, 2005, doi : 10.3180 / REACT_1477.1 .