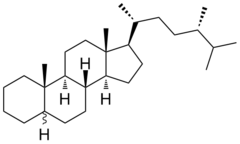
Ergostan
| Structural formula | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|||||||||||||
| General | |||||||||||||
| Surname | Ergostan | ||||||||||||
| other names |
24 S -methylcholestane |
||||||||||||
| Molecular formula | C 28 H 50 | ||||||||||||
| External identifiers / databases | |||||||||||||
|
|||||||||||||
| properties | |||||||||||||
| Molar mass | 386.71 g mol −1 | ||||||||||||
| safety instructions | |||||||||||||
|
|||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | |||||||||||||
Ergostane is a polycyclic aliphatic hydrocarbon and is one of the basic components of steroids . It consists of a tetracyclic skeleton with three fused cyclohexane rings and one fused cyclopentane ring. It differs from the Gonan in that it has two additional methyl groups and a branched nonyl group . It differs from campestane (24 R -methylcholestane) in the stereochemistry of the methyl group in the side chain.
Web links
Commons : Ergostan - collection of images, videos and audio files
Individual evidence
- ↑ This substance has either not yet been classified with regard to its hazardousness or a reliable and citable source has not yet been found.