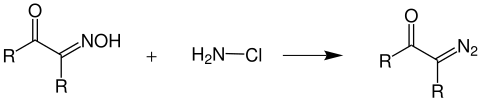Forster diazoketone synthesis
The Forster diazoketone synthesis is a reaction from the field of organic chemistry . It was discovered by Martin Onslow Forster in 1915 and named after him. The reaction is used for the synthesis of diazoketones . These can be represented by reacting α-ketooximes with chloramine .
Overview reaction
Originally this method was developed for the production of 3- diazoamphores . It was later found, however, that this reaction is generally applicable to the synthesis of α-diazoketones, which are not accessible by the previously known methods such as the Bamford-Stevens reaction . It is mainly used to make cyclic diazoketones.
An α-oximoketone (left) reacts with chloramine to form an α- diazoketone (right).
Reaction mechanism
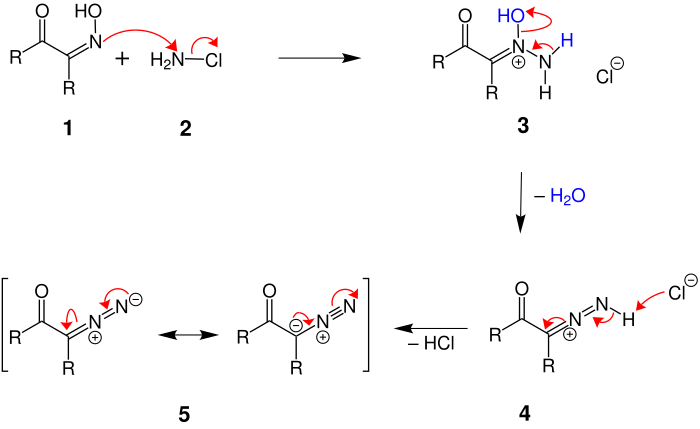
Two different reaction mechanisms are possible. A nucleophilic attack by the chloramine on the nitrogen atom of the oxime is conceivable. This variant would ascribe a major role to the carbonyl function of the educt. Meinwald et al. Showed in 1959, however, that the keto group of the starting material plays no role in the conversion of an α-ketooxime into an α-diazoketone. Therefore, the following reaction mechanism was preferred:
First there is a nucleophilic substitution of the nitrogen atom of oximoketone 1 on the nitrogen atom of chloramine 2 . The chloride ion is split off and 3 is formed . In the next step, water is split off with the formation of a nitrogen-nitrogen double bond , so that 4 is formed. In the third and last reaction step, a hydrogen atom is split off, which allows a triple bond to form between the nitrogen atoms. The result is an α-diazoketone 5 , which - as shown in the figure - has mesomeric boundary structures.
See also
Individual evidence
- ↑ a b c d Z. Wang (Ed.): Comprehensive Organic Name Reactions and Reagents (3 Volume Set). John Wiley & Sons, Hoboken, New Jersey 2009, ISBN 978-0-471-70450-8 , p. 1111.
- ^ Martin Onslow Forster: XXIX-Azotisation by chloroamine. In: Journal of the Chemical Society, Transactions. 107, 1915, p. 260, doi : 10.1039 / CT9150700260 .
- ^ A b Wolfgang Uhl, Apostolos Kyriatsoulis: Name and keyword reactions in organic chemistry. Vieweg, Braunschweig 1984, ISBN 978-3-528-03581-5 , pp. 60/61.