Glycol bath
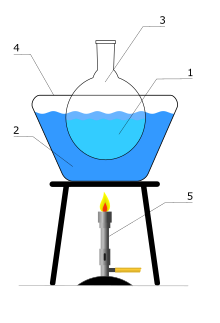
The glycol bath , also called glycol bath , consists of a container filled with warm or hot polyethylene glycol (or similar substances), in which there is another container with the substance to be heated. Glycolbäder are universally applicable and are used during the heating of substances or reaction mixtures in chemical laboratories for heating up to 150 (200) ° C . Below about 95 ° C , water baths are usually used because of the high heat capacity of the water and the resulting inertia and easy controllability. Above 200 ° C , silicone oil baths are more suitable than glycol baths.
Glycol baths have the advantage over silicone oil baths that inadvertently dripping water is not a source of danger and after the experiment, glycol adhering to the flask can easily be washed off with water.
Individual evidence
- ↑ a b Organikum . 23rd edition. Wiley-VCH Verlag, 2009, ISBN 978-3-527-32292-3 , p. 15.