Grignard degradation
The Grignard degradation is a name reaction of organic chemistry and was discovered in 1934 by the German chemist Wilhelm Steinkopf (1879–1949). The name of the degradation reaction can be traced back to Victor Grignard (1871-1935), because it is related to the Grignard reaction . The Grignard degradation is a dehalogenation of polyhalogen compounds, whereby one compound with one less halogen atom is formed.
Overview reaction
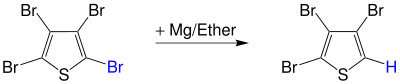
The polyhalogen compound, such as, for example, 2,3,4,5-tetrabromothiophene, forms a Grignard compound with magnesium in diethyl ether , which reacts less when mixed with water to form a polyhalogen compound with a halogen atom. In this case 2,3,4-tribromothiophene is formed and magnesium bromohydroxide is split off:
In addition to brominated starting materials, chlorinated or fluorinated aromatic compounds are used.
mechanism
A reaction mechanism has not yet been fully clarified, but can be shown using the example of 2,3,4,5-tetra bromothiophene 1 .
In the first step, an exchange of electrons takes place on the magnesium surface, as the magnesium atom transfers an electron to the bromine, thus creating a radical anion 2 . Due to the weak bond between the carbon and bromine, the radical anion breaks down into a thiophenyl radical 3 and a bromide ion. The latter reacts with the magnesium radical to form a magnesium bromide radical. Now the two radicals can combine to form a Grignard reagent 4 . The next step is to add water. The oxygen atom of the water attacks the positively polarized magnesium atom in the Grignard compound with its free electrons . At the same time, a hydrogen atom in the water is attracted to the negatively polarized carbon atom and an intramolecular proton migration takes place. After the magnesium bromohydroxide has been split off, the desired product 2,3,4-tribromothiophene 5 is formed .
See also
Individual evidence
- ^ Zerong Wang: Comprehensive Organic Name Reactions and Reagents . John Wiley & Sons, 2009, ISBN 978-0-471-70450-8 , pp. 1271-1272 .
- ^ W. Steinkopf, H. Jacob, H. Penz: Studies in the thiophen series. XXVI. Isomeric bromothiophenes and the constitution of the thiophenedisulfonic acids . In: Liebig's annals . tape 512 , 1934, pp. 136-146 , doi : 10.1002 / jlac.19345120113 .
- ↑ M. Windholz, S. Budavari, LY Stroumtsos, MN Done: The Merck Index - An Encyclopedia of Chemicals and Drugs . Merck & Co., 1976, ISBN 0-911910-26-3 , pp. ONR-38 .
- ^ W. Steinkopf, H. Jacob, H. Penz: Studies in the thiophen series. XXVI. Isomeric bromothiophenes and the constitution of the thiophenedisulfonic acids . In: Liebig's annals . tape 512 , 1934, pp. 136-146 , doi : 10.1002 / jlac.19345120113 .
- ↑ Reinhard Brückner : reaction mechanisms. 3rd corrected edition. Spektrum Akademischer Verlag, 2007, ISBN 978-3-8274-1579-0 , p. 774.
- ^ Zerong Wang: Comprehensive Organic Name Reactions and Reagents . John Wiley & Sons, 2009, ISBN 978-0-471-70450-8 , pp. 1271-1272 .