Bromothiophenes
| Monobromothiophenes | |||||||
| Surname | 2-bromothiophene | 3-bromothiophene | |||||
| other names | α-bromothiophene 2-thienyl bromide |
β-bromothiophene 3-thienyl bromide |
|||||
| Structural formula | 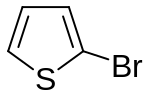 |

|
|||||
| CAS number | 1003-09-4 | 872-31-1 | |||||
| PubChem | 13851 | 13383 | |||||
| Molecular formula | C 4 H 3 BrS | ||||||
| Molar mass | 163.04 g mol −1 | ||||||
| Physical state | liquid | ||||||
| description | colorless, clear, smelly liquid |
light brown, clear, smelly liquid |
|||||
| Melting point | −10 ° C | <−10 ° C | |||||
| boiling point | 149-151 ° C | 150 ° C | |||||
| Flash point | 52 ° C | 52 ° C | |||||
| density | 1.684 g / cm 3 (25 ° C) | 1.74 g / cm 3 (25 ° C) | |||||
| Vapor pressure | |||||||
| solubility | not miscible with water | ||||||
| Refractive index | 1.586 (20 ° C) | 1.591 (20 ° C) | |||||
|
GHS labeling |
|
|
|||||
| H and P phrases | 226-300-318 |
226-301-310-317 319-330-335 |
|||||
| no EUH phrases | no EUH phrases | ||||||
|
264-280-301 + 310 305 + 351 + 338 |
260-280-284-302 + 350 305 + 351 + 338-310 |
||||||
| LD 50 | 35 mg kg −1 (oral, rat) | 66–160 mg kg −1 (oral, rat) | |||||
Bromthiophenes is the collective term for two isomeric chemical compounds that belong to the heterocycles .
presentation
2-bromothiophene
The direct bromination of thiophene with elemental bromine yields not only 2-bromothiophene but also considerable amounts of 2,5-dibromothiophene . If the bromination is carried out with potassium bromate and hydrogen bromide , only simple substitution takes place .
3-bromothiophene
3-Bromothiophene can be prepared from 2,3,5-tribromothiophene , which is easily accessible by direct bromination of thiophene, by debromination with zinc dust in acetic acid .
Reactions
When 2-bromothiophene is treated with sodium amide in liquid ammonia , isomerization to 3-bromothiophene takes place.
The isomerization can with good yields with zeolite - catalysts are performed.
use
3-bromothiophene is the most important starting material for the synthesis of 3-substituted thiophenes.
Individual evidence
- ↑ a b c d e f g data sheet 2-bromothiophene from Sigma-Aldrich , accessed on March 16, 2011 ( PDF ).
- ↑ a b c d e f g Data sheet 3-bromothiophene from Sigma-Aldrich , accessed on March 16, 2011 ( PDF ).
- ↑ a b Entry on 2-bromothiophene at ChemBlink , accessed on March 16, 2011.
- ^ Entry on 3-bromothiophene in ChemicalBook , accessed on September 19, 2011.
- ↑ Entry on 3-bromothiophene at ChemBlink , accessed on March 16, 2011.
- ↑ YL Goldfarb, AA Dudinov, VP Litvinov: "New method for preparation of 2-bromothiophene," in Russian Chemical Bulletin , 1982 , 31 (10) pp 2104 to 2105; doi : 10.1007 / BF00950665 .
- ^ A b A. R. Katritzky: Advances in Heterocyclic Chemistry , Verlag Academic Press, 1963 , ISBN 978-0-12020601-8 , p. 41 ( limited preview in the Google book search).
- ↑ a b S. Gronowitz: “New Syntheses of 3-Bromothiophene and 3,4-Dibromothiophene”, in: Acta Chemica Scandinavica , 1959 , 13 , pp. 1045-1046; doi : 10.3891 / acta.chem.scand.13-1045 ; Full text (PDF file; 271 kB).
- ↑ S. Gronowitz, T. Raznikiewicz: 3-Bromothiophene In: Organic Syntheses . 44, 1964, p. 9, doi : 10.15227 / orgsyn.044.0009 ; Coll. Vol. 5, 1973, p. 149 ( PDF ).
- ↑ L. Brandsma, RLP de Jong: “A Large-Scale Procedure for the Preparation of 3-Bromothiophene from 2-Bromothiophene and Sodamide in Liquid Ammonia”, in: Synthetic Communications , 1990 , 20 (11), pp. 1697-1700 ; doi : 10.1080 / 00397919008053091 .
- ↑ C. Werner, A. Kanschik-Conradsen, B. Kellermeier, H.-J. Schmidt: "Process for isomerization of 2-halothiophene to 3-halothiophene", United States Patent 7208610. Full text





