Compressibility factor
The compressibility factor ( symbol : z or Z ), also compression or real gas factor , not to be confused with compressibility , is a term used in thermodynamics and is used to describe the deviation of a real gas from an ideal behavior.
The compressibility factor is often used to develop the equation of state of real gases in the dimensionless representation.
description
The real gas factor is inserted as an additional factor in the thermal equation of state of ideal gases in order to describe the behavior of real gases:
It is thus defined as:
or:
The individual symbols stand for the following quantities :
- p - pressure
- V - volume
- R - universal gas constant
- T - absolute temperature
- n - amount of substance
- v - specific volume
- - specific gas constant
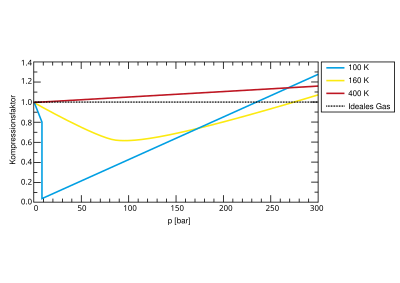
Real gases behave approximately ideally only at low pressure and high temperatures and at a volume that tends towards infinity, i.e. in accordance with the equation of state of ideal gases with a compressibility factor of one. At higher pressures, gases sometimes deviate considerably from the ideal behavior, since both forces of attraction and repulsion between the particles become noticeable. Therefore the compression factor depends on the pressure and the temperature of the gas (the molar volume in turn depends solely on the temperature). At high pressure, a real gas is generally more difficult to compress than an ideal gas, which equates to a compressibility factor greater than one. Before that, however, a minimum is passed through when the pressure increases (see figure).
At the critical point of the gas, one speaks of the critical compressibility factor or critical coefficient (see Van der Waals equation ).
Practically technical applications
Roughly (ideally calculated, i.e. assuming ideal behavior of the gas), a gas cylinder (at ambient temperature) with gas under pressure (permanent gas that has not liquefied) contains an amount of gas that corresponds to the internal volume of the cylinder times the pressure of the contents .
The usual 50-liter gas bottle for technical gases is filled with 200 bar and contains 50 x 200 = 10,000 liters = 10 (standard) cubic meters of gas when it - removed - expands to normal pressure of around 1 bar.
In fact, the delivery quantity and yield differ significantly depending on the type of gas and pressure. This is important for planning the gas supply for a dive or when filling many or large balloons with helium in order to achieve a certain buoyancy.
Divers calculate with the following densities and corrections:
Air 1 bar 1.168 kg / m3 0%
Air 100 bar 117.8 kg / m3 -1.8%
Air 200 bar 226.6 kg / m3 +3.1%
Air 300 bar 316.3 kg / m3 + 10.7% correction value = necessary addition to the nominal amount of gas to be carried in order to achieve the desired productivity.
Accordingly, air can be compressed increasingly less efficiently than ideal at pressures above about 150 bar.
Gas suppliers indicate the yield of gas cylinders in catalogs as gas quantities in standard cubic meters:
50 L x 200 bar helium 9.1 m3 oxygen 10.6 m3 nitrogen 9.6 m3 hydrogen 8.9 m3
50 L x 300 bar helium 13.2 m3 oxygen 15.2 m3 nitrogen 13.2 m3 hydrogen 12.6 m3
literature
See also
Web links
Individual evidence
- ↑ values gases seveke.de, updated April 21, 2019, accessed July 3, 2020.