Korte rearrangement
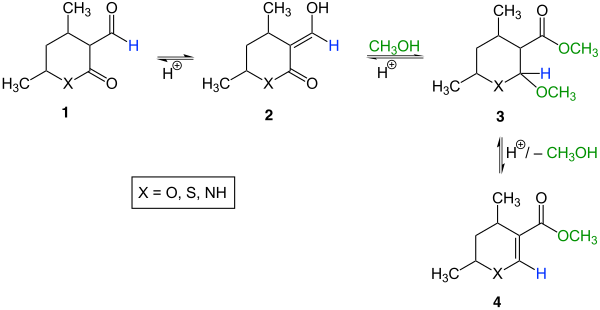
In organic chemistry, the Korte rearrangement is a rearrangement of α-acyllactones and related systems named after the German chemist Friedhelm Korte (1923–2013). So z. B. rearrange the aldehyde 1 (X = O) via the enol 2 (X = O) in hydrochloric acid methanol into the carboxylic acid esters 3 (X = O) and 4 (X = O):
The Korte rearrangement seems to be limited to five- and six-membered rings. The ratio of 3 : 4 depends on the substituents and the ring size. Often 3 can be converted into 4 by heating in the presence of polyphosphoric acid . If the formyl group in educt 1 has been replaced by an acyl group , the rearrangement also works.
Individual evidence
- ^ Hermann Höver: Reaction Mechanisms of Organic Chemistry, Verlag Chemie - John Wiley & Sons, 1973, pp. 371–376, ISBN 3-527-25442-0 .
- ^ Hermann Höver: Reaction Mechanisms of Organic Chemistry, Verlag Chemie - John Wiley & Sons, 1973, p. 372, ISBN 3-527-25442-0 .