Cryptochrome
Cryptochrome (from Greek κρυπτός, kryptós, "hidden" and χρωμα, chroma, "color") are 50 to 70 kDa heavy flavoproteins , as photoreceptors can function for blue light. Although it has been known for more than a hundred years that plants react to blue light, the cryptochrome was first identified in plants by isolating a cDNA in 1993 and has since been found in animals. The precursors of these proteins in bacteria code for light-activated enzymes that are involved in DNA repair , a function that is not preserved in eukaryotes. Cryptochromes contain two non- covalently bound chromophores , the flavin adenine dinucleotide (FAD) and a light-harvesting cofactor. They play a role in maintaining the circadian rhythm in animals and plants and in magnetoreception - the perception of the earth's magnetic field in animals.
Description and function
Cryptochromes are remarkable in that they can form a flavose-quinone radical after light absorption in vitro , which absorbs light not only in the blue region of the spectrum, but also in the green, yellow and red region.
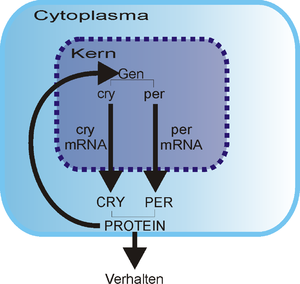
With the discovery of the CRY gene in fruit flies , which is very similar to the plant genes cry1 and cry2 and which is responsible for the production of the CRY protein, the hypothesis that cryptochromes are involved in the circadian organization became more and more probable.
Studies on knockout animals have shown that a mouse needs cryptochromes in order to ensure normal functioning of the circadian clock. By homologous recombination , Van der Horst et al. (1999) generated mutant mice that no longer had the cry1 , cry2 or both genes. The mutants behaved like normal mice under 12 hours of light and 12 hours of darkness (LD 12:12), that is, they were rhythmic in their expressions of life. However, under constant conditions (24 hours of darkness - DD) they showed arrhythmic behavior. From this one could conclude that the cryptochromes were apparently essential for normal clock function. It is not yet known how cryptochromes interact with other known mouse clock molecules such as Clock , Period or Timeless or how the molecular basis of the clock is influenced in these mutant mice. CRY1 and CRY2 have been found in mutant mice both in the eyes and in the suprachiasmatic nucleus (SCN), the location of the master clock . Therefore, one might assume that cryptochromes are responsible for light detection in mammals. However, the facts known to date speak against the participation of the cryptochromes in light detection and at least in favor of the presence of another group of photopigments, the opsins .
For migratory birds , scientists were able Oldenburg University also Cry1 and CRY2 proteins detected in the retina ( Garden Warbler ). Here, the proteins are concentrated in special cell types in the retina , which play a role especially in migrating birds. then when the garden warbler primarily orientates itself magnetically. The results of the Oldenburg Group supports the hypothesis that the Cryptochrome could be the magneto-sensory molecule that translates the magnetic information into visual signals and therefore it allows the bird, using their magnetic sense the Earth's magnetic field to perceive the earth.
literature
- Aziz Sancar : Cryptochrome: The Second Photoactive Pigment in The Eye and Its Role in Circadian Photoreception. In: Annual Review of Biochemistry . Vol. 69, 2000, pp. 31-67, doi: 10.1146 / annurev.biochem.69.1.31 , PMID 10966452 .
- Íbrahim Halil Kavaklı, Aziz Sancar : Circadian Photoreception in Humans and Mice. In: Molecular Interventions. Vol. 2, No. 8, 2002, pp. 484-492, doi: 10.1124 / mi.2.8.484 , PMID 14993400 .
- Russell N. Van Gelder: Tales from the Crypt (ochromes). In: Journal of Biological Rhythms. Vol. 17, No. 2, 2002, pp. 110-120, doi: 10.1177 / 074873002129002401 , PMID 12002158 .
- Chentao Lin, Dror Shalitin: Cryptochrome Structure and Signal Transduction. In: Annual Review of Plant Biology . Vol. 54, 2003, pp. 469-496, doi: 10.1146 / annurev.arplant.54.110901.160901 , PMID 14503000 .
- Qing-Hua Li, Hong-Quan Yang: Cryptochrome Signaling in Plants. In: Photochemistry and Photobiology. Vol. 83, No. 1, 2007, pp. 94-101, doi: 10.1562 / 2006-02-28-IR-826 , PMID 17002522 .
Individual evidence
- ↑ M. Ahmad, AR Cashmore: HY4 gene of A. thaliana encodes a protein with characteristics of a blue-light photoreceptor. In: Nature. Volume 366, Number 6451, November 1993, pp. 162-166, doi : 10.1038 / 366162a0 , PMID 8232555 .
- ^ S. Weber: Light-driven enzymatic catalysis of DNA repair: a review of recent biophysical studies on photolyase. In: Biochimica et Biophysica Acta . Volume 1707, Number 1, February 2005, pp. 1-23, doi : 10.1016 / j.bbabio.2004.02.010 , PMID 15721603 (review).
- ↑ Christopher T. Rodgers, PJ Hore: Chemical magnetoreception in birds: the radical pair mechanism. In: Proceedings of the National Academy of Sciences . Vol. 106, No. 2, 2009, pp. 353-360, doi : 10.1073 / pnas.0711968106 .
- ↑ A. Günther, A. Einwich, E. Sjulstok, R. Feederle, P. Bolte, KW Koch, IA Solov'yov, H. Mouritsen: Double-Cone Localization and Seasonal Expression Pattern Suggest a Role in Magneto Reception for European Robin Cryptochrome 4. In: Current biology: CB. Volume 28, Number 2, January 2018, pp. 211-223.e4, doi : 10.1016 / j.cub.2017.12.003 , PMID 29307554 .
- ↑ T. Todo, H. Ryo, K. Yamamoto, H. Toh, T. Inui, H. Ayaki, T. Nomura, M. Ikenaga: Similarity among the Drosophila (6-4) photolyase, a human photolyase homolog, and the DNA photolyase-blue-light photoreceptor family. In: Science. Volume 272, Number 5258, April 1996, pp. 109-112, PMID 8600518 .
- Jump up ↑ GT van der Horst, M. Muijtjens, K. Kobayashi, R. Takano, S. Kanno, M. Takao, J. de Wit, A. Verkerk, AP Eker, D. van Leenen, R. Buijs, D. Bootsma , JH Hoeijmakers, A. Yasui: Mammalian Cry1 and Cry2 are essential for maintenance of circadian rhythms. In: Nature. Volume 398, Number 6728, April 1999, pp. 627-630, doi : 10.1038 / 19323 , PMID 10217146 .
- ↑ H. Mouritsen, U. Janssen-Bienhold, M. Liedvogel, G. Feenders, J. Stalleicken, P. Dirks, R. Weiler: Cryptochromes and neuronal-activity markers colocalize in the retina of migratory birds during magnetic orientation. In: Proceedings of the National Academy of Sciences . Volume 101, number 39, September 2004, pp. 14294-14299, doi : 10.1073 / pnas.0405968101 , PMID 15381765 , PMC 521149 (free full text).
