Membrane distillation
In the membrane distillation is a thermally-driven separation process in which the separation due to a phase change takes place. A hydrophobic membrane represents a barrier for the liquid phase (e.g. salt water) of a fluid flow, but the vapor phase (e.g. water vapor) can permeate through the pores of the membrane . The driving force for the process is a partial vapor pressure gradient, which is usually caused by a temperature difference.
Principle of membrane distillation
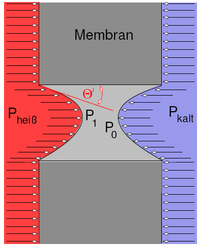
In the usual separation processes, in which the mass flows are separated by a membrane, the driving force between the two interfaces is a static pressure difference (e.g. reverse osmosis ), a concentration gradient ( dialysis ) or an electric field ( ED ). The selectivity of the corresponding membrane is caused by its pore size in relation to the size of the substance to be retained , its diffusion coefficient or its electrical polarity. The selective property of a membrane that is used for membrane distillation (MD), however, is based on the retention of liquid water with simultaneous permeability for free water molecules d. H. Steam. These membranes are made of a hydrophobic plastic (e.g. PTFE, PVDF or PP) and have pores with a diameter of usually 0.1 to 0.5 µm. Since water has strong dipole properties, while the membrane material is non-polar, there is no wetting of the material by the liquid. Although the pores are significantly larger than the molecule, the high surface tension of water prevents the liquid phase from penetrating the pores, with a convex meniscus forming into the pore. This effect is known as capillary depression . The depth of penetration depends, among other things, on the external pressure that is on the liquid. A measure of the penetration of the liquid into the pores is the contact angle Θ = 180 - Θ 'As long as: Θ> 90 ° or Θ'> 0 ° there is no wetting of the pore. If the external pressure is greater than the so-called wetting pressure, then Θ = 90 ° and there is a short circuit in the pore. The driving force that conveys the vapor through the membrane in order to be able to recover it as a product on the permeate side is the water vapor partial pressure difference between the two interfaces of the membrane. This partial pressure difference is the result of a temperature difference between the two interfaces. As can be seen in the adjacent figure, the membrane is exposed to a warm feedwater flow on one side and a cooled permeate flow on the other. The temperature difference across the membrane, which is usually in the range of 5 to 20K, brings about a corresponding partial pressure difference, which ensures that the water vapor generated at the membrane interface permeates through the membrane pores following the pressure gradient and condenses on the colder side.
Membrane distillation process
Various membrane distillation processes are used in technology. There are essentially four processes that differ primarily in the structure of the distillate channel and its operation. The following technologies are common:
- Direct Contact MD procedure (DCMD)
- Air Gap MD process (AGMD)
- Vacuum MD process (VMD)
- Sweeping Gas MD method (SGMD)
Direct Contact MD
In the DCMD process, both sides of the membrane are exposed to liquid. The hot feed water is on the evaporator side, while the permeate side is cooled. The condensation of the vapor permeating through the membrane takes place directly in the liquid phase at the membrane boundary layer. Since in this case only the membrane acts as an obstacle to the transport of substances, relatively high area-specific permeate flows can be achieved here. A disadvantage, however, is that the resistance offered by the membrane is also low for the sensible heat conduction, which results in a relatively high loss of heat from the evaporator to the condenser. This amount of heat is not available to the distillation process, which makes the process less efficient.
Air Gap MD
In the Air Gap MD process, the structure of the evaporator channel corresponds to that of the DCMD process, while the permeate space lies between the permeate side of the membrane and a cooled wall and is filled with air. The water vapor permeating through the membrane must also overcome this air gap before it condenses on the colder wall. The advantage of this method is that the air gap forms good thermal insulation from the capacitor, which means that heat conduction losses can be significantly reduced. The disadvantage, however, is that the air gap represents an additional resistance for the transport of the substance, which means that the area-specific permeate output is reduced compared to the DCMD process. Another advantage of the AGMD over the DCMD is that volatile substances with low surface tension such as B alcohol or other solvents can be separated from aqueous solutions, since with AGMD there is no contact between the liquid permeate and the membrane, which would result in their wetting.
Sweeping Gas MD
With the Sweeping Gas MD, also called air stripping in technology, a channel structure with a free gap on the permeate side is used; this corresponds to the structure with the AGMD. At the SGMD, this gap is flushed with a gas that entrains the vapor that has permeated through the membrane and carries it out of the module. The condensation of the steam takes place on a condenser located outside the MD module. As with AGMD, volatile substances with low surface tension can also be distilled with this process. The advantage of SGMD compared to AGMD is that the transfer resistance of the air gap, which inhibits the transport of substances, can be significantly reduced by the forced flow. This enables a significantly higher area-specific material flow to be achieved than with AGMD. The disadvantage of the SGMD is that higher condenser capacities are required due to the gas content and the associated total mass flow. When using low gas mass flows, on the other hand, there is the risk that the gas on the warmer membrane will heat up too much and thereby reduce the partial pressure difference and thus the driving force. A solution to this problem is proposed in that in the SGMD, as in the AGMD, a cooled wall of the permeate channel is used, via which the purge gas is tempered.
Vacuum MD
The Vacuum MD also uses a duct structure with an air gap. The vapor that has permeated through the membrane is drawn off from the permeate channel via a negative pressure and, as in the SWGMD process, is condensed outside of the module. Like SGMD, VMD can be used to separate volatile substances from an aqueous solution or to generate pure water from concentrated brine. It is advantageous that undissolved inert gases, which can block the membrane pores, are sucked off by the negative pressure and thus a larger effective membrane area is available. In addition, by reducing the boiling point, a comparable productivity is achieved even at lower absolute temperatures and lower temperature differences across the membrane. Due to the lower temperature difference to be applied, the specific thermal energy requirement is reduced. The disadvantage here is that the generation of negative pressure, which has to be set in accordance with the temperature of the brine, requires a great deal of equipment. The MD modules must be vacuum-tight and stable. The electrical energy requirement is significantly higher than with the DCMD and AGMD processes. In addition, there is the problem that the pH value rises because CO 2 is withdrawn from the feed water .
Permeate Gap MD
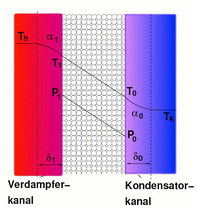
In the following, the basic channel structure and the mode of operation of a normal DCMD module and a DCMD module with a separate permeate channel will be explained. The structure shown on the right in the picture outlines a flat channel arrangement, but can be understood as a scheme for plate modules, hollow fiber modules or spiral wound modules. The channel consists of the condenser channel with inlet and outlet and the evaporator channel with inlet and outlet. The evaporator channel and condenser channel are separated from each other by the hydrophobic, microporous membrane. Fresh water flows through the condenser channel for cooling purposes, and salt-containing feed water flows through the evaporator channel, for example. The cooling water flows into the condenser duct at a temperature of, for example, 20 ° C. The water vapor permeating through the membrane condenses in the cooling water, its latent heat being released again and leading to an increase in the temperature of the cooling water. In addition, due to the sensitive conduction of heat through the membrane, heat is introduced into the cooling water. Because mass transport takes place through the membrane, the mass flow in the evaporator channel decreases while it increases by the same amount in the condenser channel. The preheated cooling water mass flow leaves the condenser duct at a temperature of 72 ° C and is fed to a heat exchanger in order to preheat the feed water. The preheated feed water is fed to a further heat source for reheating and then fed into the evaporator duct of the MD module at a temperature of 80 ° C. The formation of steam removes latent heat from the feed water, which results in further cooling in the direction of flow. In addition, heat is withdrawn from the feed water due to sensitive heat conduction through the membrane. The cooled feed water leaves the evaporator duct at around 28 ° C, which means that the temperature difference compared to the condenser inlet is the same as that between the condenser outlet and the evaporator inlet. With a PGMD module, the permeate channel is separated from the condenser channel with a condensation surface. In this case, the cooling water flowing through the condenser channel can directly be the salty feed water, since it does not come into contact with the permeate. The cooling or feed water entering the condenser at temperature T1 is now used to cool the permeate in the permeate channel. The condensation of the vapor takes place in the liquid permeate. The preheated feed water, which was used to cool the condenser, can, after leaving the condenser at temperature T2, be fed directly to a heat source for reheating and then fed to the evaporator at temperature T3. The permeate is removed at temperature T5, the cooled concentrate is removed at temperature T4. A major advantage of the PGMD process compared to the DCMD process is that the feed water can be fed directly through the module for cooling and then the total mass flow only reaches the evaporator via an external heat exchanger. This reduces heat transfer losses and saves costly components. Another advantage is that the excess permeate does not have to be withdrawn from the cooling water, since the permeate has already been separated and the cooling water mass flow in the condenser remains constant. The disadvantage is that the permeate in the permeate channel has only a minimal flow velocity and therefore the heat transfer from the membrane boundary surface to the condenser wall is very poor. This results in relatively high temperatures at the permeate-side boundary surface of the membrane (temperature polarization), which leads to a reduction in the vapor pressure difference and thus the driving force. The advantage is that the poor heat transfer reduces the losses due to heat conduction via the membrane. Compared to the AGMD, a higher area-specific permeate flow is achieved because the material flow is not additionally slowed down by the diffusion resistance of an air layer.
Applications
Typical applications of membrane distillation are / can be:
- Seawater desalination
- Brackish water desalination
- Process water treatment
- Ultrapure water production
- Ammonium removal / concentration
- Material flow recycling
- Recyclable concentration
Solar powered membrane distillation
Membrane distillation, especially in the form of a spiral wound module based on a GORE patent from 1985, is ideally suited for compact, completely solar-powered desalination systems for small to medium daily capacities ≤ 10,000 l / day. As part of the EU project MEMDIS, which began in 2003, the Fraunhofer Institute for Solar Energy Systems (ISE) and project partners began to design MD modules and to develop, install and examine two different solar-powered systems. The first system is a system known as a compact system for the production of approx. 100–120 l / day of drinking water from sea or brackish water. The main objective of this system design was to create a simply structured, energy self-sufficient, maintenance-free and robust system for target markets in areas with poor infrastructure in arid and semi-arid zones. The second system was called a two-circuit system with a capacity of about 2,000 l / day. The collector circuit was decoupled from the desalination circuit by a saltwater-resistant heat exchanger. Based on these two systems, several prototype systems were developed, installed and measured.
With the standard configuration of today's (2011) compact system, a distillate yield of up to 150 l / day can be achieved. The thermal energy is provided by a 6.5 m² solar thermal collector field, the electrical energy by a 75 W PV module. The system is being developed and marketed by Solar Spring GmbH, a spin-off from the Fraunhofer Institute for Solar Energy Systems ISE. As part of another EU project, the MEDIRAS project, a further developed two-circuit system was set up on Gran Canaria in 2011. The system is installed in a 20 ft shipping container and, with a collector area of 225 m² and a heat storage unit, enables distillate yields of up to 3,000 l / day. Further applications with up to 5,000 l / day have been implemented, whereby the process is either 100% solar-powered or operated as a hybrid process in combination with waste heat.
Challenges in membrane distillation
Although membrane distillation is potentially attractive for some applications, it still suffers from some disadvantages and has found little acceptance in the industry. These disadvantages include high energy consumption compared to alternative membrane processes and wetting phenomena. The energy demand for membrane distillation can be met if it is integrated with renewable energy or available "waste heat" and new configurations and operating conditions further improve the energy efficiency of membrane distillation. However, the occurrence of membrane pore wetting due to the loss of membrane hydrophobicity in feeds containing wetting agents (e.g. oils, surfactants) continues to question their industrial potential.
Approaches to avoid wetting
Several researchers have proposed different approaches to controlling wetting in membrane distillation. The main focus was on the further development of membrane production in order to ensure a low affinity between the liquid and the polymer material. This was mainly achieved by modifying the geometric structure of the membrane surface and the surface chemistry. Several studies also examined the integration of filtration processes as pre-treatment steps for membrane distillation. To control the wetting, operating conditions and novel flow techniques such as the recharging of an air layer on the membrane surface are used in further studies.
activities
Several companies and research institutes around the world work and research with and on the process of membrane distillation. Are currently active u. a. the following:
- Fraunhofer Institute for Solar Energy Systems ISE, Freiburg, Germany
- SolarSpring GmbH, Freiburg, Germany
- Keppel Seghers
- Scarab Development AB, Sweden
- Plataforma Solar de Almería, Spain
- ITM-CNR. Istituto per la Tecnologia delle Membrane, Italy
- Instituto Tecnológico de Canarias, SA, Spain
- Università Degli Studi Di Palermo, Italy
- Deukum GmbH, Frickenhausen, Germany
- Institute for Environmental Process Engineering, University of Bremen, Germany
- memsys clearwater Pte. Ltd., Singapore and Grafing near Munich
- AEE INTEC, Institute for Sustainable Technologies, Austria
- Institute for Process Engineering, Johannes Kepler University Linz, Austria
See also
Individual evidence
- ↑ a b c Joachim Koschikowski: Development of energy self-sufficient working water desalination plants based on membrane distillation. Fraunhofer Verlag, 2011, ISBN 978-3-8396-0260-7 .
- ↑ SolarSpring GmbH
- ↑ Mohammad Rezaei, David M. Warsinger, John H. Lienhard V, Mikel C. Duke, Takeshi Matsuura: Wetting phenomena in membrane distillation: Mechanisms, reversal, and prevention . In: Water Research . tape 139 , August 2018, p. 329–352 , doi : 10.1016 / j.watres.2018.03.058 ( elsevier.com [accessed on August 24, 2020]).
- ↑ David Warsinger, Karan Mistry, Kishor Nayar, Hyung Chung, John Lienhard: Entropy Generation of Desalination Powered by Variable Temperature Waste Heat . In: Entropy . tape 17 , no. 11 , October 30, 2015, ISSN 1099-4300 , p. 7530-7566 , doi : 10.3390 / e17117530 ( mdpi.com [accessed August 24, 2020]).
- ^ Edward K. Summers, John H. Lienhard: Experimental study of thermal performance in air gap membrane distillation systems, including the direct solar heating of membranes . In: Desalination . tape 330 , December 2013, p. 100–111 , doi : 10.1016 / j.desal.2013.09.023 ( elsevier.com [accessed on August 24, 2020]).
- ↑ Mohammad Rezaei, Wolfgang Samhaber: Wetting behavior of superhydrophobic membranes coated with nanoparticles in membrane distillation . In: Chemical Engineering Transactions . tape 47 , March 2016, p. 373-378 , doi : 10.3303 / CET1647063 .
- ↑ Mohammad Rezaei, David M. Warsinger, John H. Lienhard V, Wolfgang M. Samhaber: Wetting prevention in membrane distillation through superhydrophobicity and recharging an air layer on the membrane surface . In: Journal of Membrane Science . tape 530 , May 2017, p. 42–52 , doi : 10.1016 / j.memsci.2017.02.013 ( elsevier.com [accessed August 24, 2020]).
- ↑ Mohammad Rezaei, David M. Warsinger, John H. Lienhard V, Wolfgang M. Samhaber: Wetting prevention in membrane distillation through superhydrophobicity and recharging an air layer on the membrane surface . In: Journal of Membrane Science . tape 530 , May 2017, p. 42–52 , doi : 10.1016 / j.memsci.2017.02.013 ( elsevier.com [accessed August 24, 2020]).
literature
- HE Hoemig: Seawater and Seawater Distillation Vulkan-Verlag, 1978, ISBN 3-8027-2438-0 .
- D. Winter, J. Koschikowski, M. Wieghaus: Desalination using membrane distillation: Experimental studies on full scale spiral wound modules. Fraunhofer ISE, Freiburg 2011.