Schottky defect
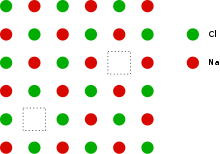
Schottky defects (also: Schottky disorder ) are among the lattice defects in crystal lattices . They are intrinsic point defects in which pairs of vacancies occur in an ion lattice . In the simplest case, one ion is missing in the anion sublattice and in the cation sublattice . The disorder is named after Walter Schottky . Occasionally a single void in an ion lattice is referred to as a Schottky defect. In the case of a Frenkel defect , an ion has moved from a regular lattice site to an interstitial lattice site, leaving a void behind. Frenkel and Schottky defects are called intrinsic defects that do not change the stoichiometry of a crystal.
description
The Schottky disorder is generated by the fact that ions close to the surface leave their lattice position, migrate to the surface of the crystal and accumulate there. The resulting voids can move through vacancy migration in the crystal. They can therefore make a significant contribution to the mass transport and reactivity of a solid.
Schottky defects are a natural property of many ion crystals, that is, they are in chemical equilibrium . They mainly occur in connections in which a Frenkel disorder is difficult due to the lack of suitable interstitial spaces .
Examples of such crystals are the alkali halides such as sodium chloride and potassium chloride . The concentration (“ defect concentration ”) of the voids can be formally described using a kind of law of mass action . The number of Schottky defects increases with increasing temperature, because they increase the entropy (they are energetically unfavorable). The density of the Schottky defects in a crystal is proportional to , with as Boltzmann constant and as the material-dependent formation energy of the Schottky defects.
Individual evidence
- ↑ Walter J. Moore: Fundamentals of physical chemistry . Walter de Gruyter, 1990, ISBN 978-3-11-009941-6 , p. 753 ( books.google.de ).
- ↑ C. Kittel: Introduction to Solid State Physics. 7th edition, Oldenbourg, 1986, ISBN 3-486-20240-5 , p. 584 ff.
- ^ AF Holleman , E. Wiberg , N. Wiberg : Textbook of Inorganic Chemistry . 101st edition. Walter de Gruyter, Berlin 1995, ISBN 3-11-012641-9 , p. 1621.