Secosteroids

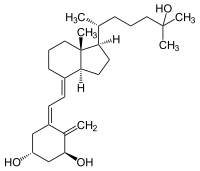
Secosteroids are a group of naturally occurring chemical compounds that are derived from steroids . They include some important substances from the vitamin D group. The physiologically active form of the D vitamins is calcitriol , which acts as a protein inductor , immune system modulator and calcium absorption in the intestine.
Structure and examples
Steroids consist of four combined ring structures as ring A to the ring D are designated. During the formation of secosteroids in organisms, the B-ring of 7-dehydrocholesterol is usually opened by UV radiation . This photolysis initially produces previtamin D 3 , which spontaneously rearranges ( isomerizes ) to form cholecalciferol . Analog arises from ergosterol the ergocalciferol ( vitamin D 2 ). From these, further secosteroids can be produced by UV light irradiation or other reactions. Since most secosteroids have ring B opened at the 9-10 position, they are more precisely called 9,10-secosteroids .
The best- known examples are cholecalciferol (vitamin D 3 ) and ergocalciferol (vitamin D 2 ), calcidiol , calcitriol , tachysterol , lumisterol and tacalcitol .
literature
- Klaus Pietrzik, Ines Golly, Dieter Loew, Handbook Vitamins: For Prophylaxis, Therapy and Advice , Urban & Fischer, 2007
Web links
- Faustino R. Pérez-López: Vitamin D: The secosteroid hormone and human reproduction , Gynecological Endocrinology, 2007
Individual evidence
- ↑ a b Wissenschaft-Online-Lexika: Entry on steroids in the Lexikon der Biochemie, accessed on November 22, 2009.
- ↑ a b H.-D. Belitz, W. Grosch, P. Schieberle: Textbook of food chemistry. 6th edition, 2007, Springer, ISBN 978-3-540-73201-3 , p. 415.