Quadruple bond
A quadruple bond is a rare form of chemical bond between two atoms that is mediated via electron pairs ( electron pair bond ). Four pairs of binding electrons between the atoms ensure the cohesion of the molecule built on them . A quadruple bond thus has a particularly high electron density and is a center of negative charge .
Examples
A known example of the quadruple bond is the group of Octahalodirhenat - complex ions Re 2 X 8 2- . In addition to the best known of these, the octachloridodirhenate (III) ion (Re 2 Cl 8 2− ), first described as a quadruple bond in 1964 by Frank Albert Cotton , bromine , iodine and - very rarely - fluorine complexes are currently known.
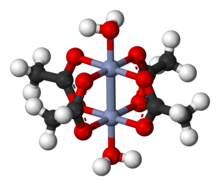
Another compound with a quadruple bond is the deep red dichromium (II) tetraacetate dihydrate Cr 2 (CH 3 COO) 4 (OH 2 ) 2 , which is relatively stable for a chromium (II) compound. It was first synthesized by Eugène-Melchior Péligot in 1844, but its character as a quadruple bond was not discovered until much later.
See also
literature
- Wolfgang A. Herrmann, Fritz E. Kühn: Organorhenium Oxides . In: Accounts of chemical research . tape 30 , no. 4 , 1997, p. 169-180 , doi : 10.1021 / ar9601398 .
- FA Cotton, RA Walton: Multiple Bonds Between Metal Atoms , Oxford University Press 1993.
Individual evidence
- ↑ FA Cotton, CB Harris: The Crystal and Molecular Structure of Dipotassium Octachlorodirhenate (III) Dihydrate, K2 [Re2Cl8] · 2H2O . Inorg. Chem., Vol. 4, 1965, pp. 330-333.
- ↑ In 1963, Soviet scientists found crystallographic evidence of a quadruple bond in this molecule, but incorrectly described the structure. VG Kuznetsov, PA Koz'min, The structure of (pyH) HReCl4 , Zhurnal Strukturnoi Khimii 1963, 4, 55-62.
- ^ Gerd Blumenthal, Dietmar Linke, Siegfried Vieth: Chemistry: Basic knowledge for engineers. 1st edition, Vieweg + Teubner, 2006, ISBN 978-3-519-03551-0 , p. 327.