Snot (disease)
| Classification according to ICD-10 | |
|---|---|
| A24 | Snot [malleus] and melioidosis [pseudorot] |
| A24.0 | Snot, infection by Burkholderia mallei, malleus |
| ICD-10 online (WHO version 2019) | |
The snot (also Muerde or skin worm , Latin Malleus ) is primarily a disease of equines ( horses and donkeys ), by which occurs mainly in Africa, Asia and South America bacterium Burkholderia mallei (synonym Malleomyces mallei caused). Humans and other mammals can also become infected with snot. The disease is characterized by specific, purulent- melting processes in the upper respiratory tract, the lungs and in the form of lumps, ulcers and abscesses of the skin and mucous membranes. The coursevaries between acute and chronic, with clear species differences. Horses can become infected latently and are the only natural reservoir for the pathogen to spread the disease. It is transmitted through direct or indirect contact with infectious body excretions ; frequent entry points for the pathogen are the mucous membranes of the head and the outer skin.
The zoonosis snot occurs rarely in humans and is often fatal if left untreated. An antibiotic therapy designed to lengthy and sometimes difficult. Effective vaccinations do not exist. Working with the pathogen is limited to laboratories of security level 3 and higher. Because of its human pathogenicity and potentially high contagiousness , Burkholderia mallei is classified as a class B biological warfare agent .
Snot is one of the oldest known diseases in horses and has been mentioned since ancient times. As a warranty failure and formerly worldwide spread, the disease led to high losses in army horses of the 18th to 20th centuries. The notifiable animal disease is subject to strict control measures, which are primarily based on the culling of sick and infected animals. Thanks to consistently implemented eradication programs, snot has now been eradicated in North America and Western Europe, but there are endemic areas in Asia, South America and Africa. Snot is currently classified as a re-emerging disease .
meaning
Snot is considered a socio-economically significant animal disease. It is classified as a threat to public health and influences the international trade in animals or their products.
The detection of the disease usually results in the immediate culling of all infected animals. Considerable economic damage is caused not only to the animal keeper concerned , but also to the respective country through internationally imposed trade bans and costly, complex control measures. Snot is a life-threatening direct zoonosis and the pathogen is classified as "one of the most dangerous organisms that can be handled in laboratories" . The pathogen deserves special attention given its usefulness as a potential agent in biological warfare .
In the industrialized nations, the once feared and widespread horse disease has long since lost its importance due to the general decline in the horse population and targeted state snot control measures. The infection currently still plays a major role in less developed countries with high horse density. Sociocultural and structural conditions often prevent effective control of the latently infected endemic herd.
Surname
The disease name Rotz (Middle High German rotz / roz , "Schleim, Rotz, in medieval and early modern humoral pathology also phlegm, white mucus ") is derived from the nasal secretions that typically occur in this disease ( ahd. Roz , from the onomatopoeic Indo-European root * ker , * kor , * kr ; compare the sounding gr. koryza "nasal mucus", at the same time the sound of throat clearing or spitting). The name glanders , which is common in the English-speaking world , as well as the Dutch droes, refers to the word "glands", as a reference to the swollen lymph nodes or the elongated skin bulges in the affected animals. In German, however, this designation would have led to a collision with the name of the disease Druse (Coryza [!] Contagiosa equorum) - a frequent confusion or equation of the two diseases in earlier times can be assumed. Instead, snot was also called worm for the same reason , as the swellings look like snaking parasites under the skin. As a worm in the oldest examples of German language today dive forward to not just tangible ailments in animals, as in the old Saxon worm blessing ( gang uz, nesso ) .
The differentiation between diseases of the respiratory tract and that of the skin can be found not only in German but also in English ( glanders & farcy ), French ( morve & farcin ) and Italian ( morvo & farcino ). In the Spanish-speaking world, the disease is known as muermo .
The exact etymology of the Latin name malleus remains in the dark. One suspects a connection with the Latin malleus for "hammer"; In Middle Latin glosses there is a connection with the phrase “to hit with the hammer”, which could refer to the sudden onset of the disease. Some ancient authors also speak generally of morbus malleus - the "bad disease" - under which they also sum up typical snot symptoms. Probably originally from the ancient Greeks to arise corrupting readings but sounding roots would offer at the diverse way diverse origins similar to, for example male "armpit, armpit," malesos "violent, greedy, crushing," malaria-[chia] "laxity, lack of energy, Softness, weakness ”. In his Historia animalium, Aristotle describes a disease that only affects donkeys as "snot" (melida) .
In the older specialist literature one can still find the adjectives that were previously used in veterinary medicine, snotty and malleous . In addition to the names maleus and malleus , the name maliasmus is sometimes used there for the disease.
distribution
| country | Year of repayment |
|---|---|
| Australia | 1891 |
| Great Britain | 1928 |
| Switzerland | 1937 |
| United States | 1942 |
| Austria | 1952 |
| Germany | 1955 |
| Netherlands | 1957 |
| Poland | 1957 |
| France | 1965 |
The disease was present worldwide until the beginning of the 20th century. Systematic testing and culling programs have meanwhile successfully eradicated the disease in Western Europe and North America, and until 2009 the pathogen was mainly found in Africa, Asia and South America. In Germany , the last case of the domestic equine population occurred in 1955, the last case of illness in 1995, and the last case of snot disease in humans on German soil was diagnosed in 1973. At the end of January 2015, snot was officially detected in a horse in Germany for the first time in 60 years by detecting snot-specific DNA. The horse showed no clinical symptoms and the previous bacteriological examination was negative. The route of infection for this infection in a horse in Lower Saxony that was previously kept in Schleswig-Holstein is unclear. A serial examination of 70 samples did not reveal any evidence of snot infection in Schleswig-Holstein.
Between 1996 and 2003, equine snot was found in Bolivia , Brazil , Eritrea , Ethiopia , Iran , Latvia , Mongolia , Myanmar , Pakistan , Turkey and Belarus . During the same period, cases of human snot disease were reported from Cameroon , Curaçao , Sri Lanka , Turkey and the USA (laboratory infection). In 2007, outbreaks of snot in horses again became known from India ( Kashmir ) and Russia (Southeast Siberia ).
As a non rotzfrei or endemic durchseucht classification of valid according to World Organization for Animal Health (OIE) currently Iran, Brazil, India and Mongolia. In India and Brazil, the disease is limited to certain regions.
In general, a distinction must be made between countries with open outbreaks of snot and countries with exclusively silent infections ( serological prevalence ). For socio-economic reasons, consistent state surveillance and control of the malleus is not possible in all nations. The data reported to the OIE are also often incomplete. It can therefore be assumed that there are further endemic herds in Africa, East Asia, Iraq, Turkey and Eastern Europe. The globalization of horse trading and equestrianism allows any time a new introduction of the disease in rotzfreie areas. This danger was last threatened in Dubai in 2004 and in Germany in 2005 by imported horses, which, however, were still in quarantine and therefore had no contact with local animal populations.
Since the 1990s, the number of snot-sick racing, military and hobby horses in Asia and South America has risen continuously, which is why snot is considered a re-emerging disease .
etiology
The causative agent of the snot disease is the bacterium Burkholderia mallei . Although classified in the genus Burkholderia since 1993 , the former name Pseudomonas mallei z. T. still used synonymously .
Burkholderia mallei is a gram-negative rod that does not form endospores and shows slow growth on simple or compound culture media at 37 ° C under aerobic conditions . Although the bacterium no capsule has in the strict sense, it has a capsule-like structure of neutral sugars on. The lack of flagellation and the resulting immobility are an important diagnostic criterion.
In addition, Burkholderia mallei differentiates itself from other members of the genera Burkholderia and Pseudomonas through its real parasitism , which means that the pathogen occurs primarily in the infected organism. The virulence of a strain can increase after targeted transmission to highly receptive hosts (inoculation). In contrast, it is only moderately stable when exposed to the environment and is sensitive to dehydration , light and common disinfectants . While it loses its infectivity after three days in dried-up secretions, it can remain infectious for several weeks in damp, dark areas.
With the causative agent of the pseudorot , Burkholderia pseudomallei , it shows about 70% agreement in the genome . One possible thesis is that the pathogen causing snot developed from Burkholderia pseudomallei . The close relationship between the two pathogens leads to similar clinical pictures and also influences the informative value of serological diagnostics.
Epidemiology
Host spectrum
Snot is a disease of the unpaired ungulates (Perissodactyla), especially the Equidae . The main hosts for Burkholderia mallei are horses, donkeys and their crossbreeds . The zebra as a potential host can also be assumed. In the event of close contact with infected equidae, other mammalian species can become infected in addition to humans . Among the domestic animals, the camel is considered to be particularly sensitive. Cases of natural snot infections have also been described in dogs , cats, and goats .
The cats (Felidae) seem to be more receptive to carnivores . Outbreaks in large cats in captivity are known, and sporadic diseases occur in domestic cats . Even wild animals such as bears , wolves , field mice , rabbits and voles can become infected. A possible illness of elephants in the zoo is also documented. Cattle , pigs and birds are not considered to be susceptible to natural infections.
With the exception of pigs, experimental infection is possible in most domesticated animal species. Among the rodents , hamsters and guinea pigs are considered to be particularly susceptible, while house mice can only be infected by a very high dose of pathogen .
Sources of infection and routes of transmission
The infection with Burkholderia mallei is usually through the oral route ( orally ) and by skin contact - rarer than droplet infection (over the air via air ) - by infected horses. All body excretions and blood can contain pathogens. Nasal secretions , lung ejections , saliva and pus from freshly opened skin wounds are considered to be particularly infectious . Infection occurs through direct contact with animals or indirectly through contaminated objects. The shared use of cribs and drinking troughs plays a central role . Litter , cleaning supplies , dishes and poorly disinfected veterinary instruments are further sources of infection. Transmission can also take place via mating. Infection of the fruit of the womb in the mother ("vertical transmission") is possible, but rare.
The pathogen is often transmitted to humans as dirt and smear infection through direct contact with sick individuals and their excretions. Another possibility of direct infection is to work with infectious tissue or bacterial cultures . As a rule, the pathogen enters via small skin wounds. A possible penetration (penetration) intact skin is suspected, but remains unproven. Infections by inhalation ( inhalation ) as well as the mouth, eyes and nasal mucous membranes (oral, conjunctival and nasal) are possible. Infections following the consumption of meat from snot-sick animals have been reported. Transmission through sexual contact from person to person has been proven in at least two cases.
Carnivorous animals usually become infected by ingesting meat from snot-sick horses or donkeys.
Spread of the disease and reservoir of pathogens
The eradication of snot, which has been successfully carried out in many countries, shows that equidae are the only natural reservoir . The introduction is carried out by a stallung diseased or latently infected animals, the latter being significant epidemiological must be given to significance.
Since the susceptibility of the individual animal depends on the dose of infection , the route of transmission and the individual constitution, the infection shows only a slow tendency to spread under normal housing conditions. Keeping them in a confined space favors infection, which is why snot disease is one of the real stable diseases . As a rule, the pits neighbors get sick first. The transfer to other horses but by natural herd and social behavior , such as skin care, sniffing and snorting effectively promoted (so-called contact disease ). Although snot does not have a pronounced seasonal character, it can occur more frequently in the damp, cold season.
Frequent animal changes with many entries and exits increases the risk of introduction. Poor housing conditions , high performance requirements and a poor immune status are considered to be factors that promote infection ( predisposing ) and that have a negative impact on the frequency of clinically visible ( manifest ) diseases and the clinical course. Therefore clinical snot kicked in the past often heaped or even disease liable for army animals, in big cities, at Fuhrmanns- and mine horses as well as stud farms , state farms and collective farms on ( epidemics ).
The snot as a war animal disease
The wars of the 17th to 20th centuries resulted in a strong spread of snot worldwide. Mounted units were usually composed of horses of mixed origins without effective entry controls. The transport to the war zones - especially by sea - was associated with considerable stress and could already trigger the disease. The number of nursing staff and veterinarians was generally inadequate for the number of animals.
During military service, the animals' immune system was severely weakened by overexertion, injuries, infectious diseases (e.g. mange ), malnutrition , and heat and cold stress. They were housed in overcrowded, inadequate stables, and the common use of drinking troughs, buckets, troughs and other accessories was the rule. Large numbers of animals were stabled in horse depots and military hospitals , and they had to record considerable inflows and outflows. The amalgamation and exchange of troops and the delivery of replacement horses resulted in animal traffic over long distances. Thorough diagnostic examinations of the animals could not always be carried out even in the 20th century due to a lack of time, qualified personnel and optimal equipment. Contact with enemy mounted units or native equidae, as well as requisitioning of prey horses from contaminated areas, encouraged the introduction of the pathogen and further spread of the infection.
In the warring countries, however, the actual peaks of the epidemic were only recorded after the end of the armed conflict and the return of the infected animals from the front . Unnecessary animals from army stocks were often discarded and sold to the civilian population, which enormously promoted the spread of the infection, especially in the USA after the American Civil War . The shortage of horses caused by the levy or loss of animals was also covered by buying horses from other areas and favored the trade in sick or inferior animals. After the two world wars, the influx of refugees from the eastern regions contributed significantly to the increase in the local epidemic. The destruction of veterinary structures in defeated nations led to a delay in the fight against snot.
Risk of infection for humans
Since the natural transmission from horse to humans is irregular and not particularly efficient, snot epidemics in humans are unknown. Even with a disease frequency ( morbidity ) of 5–30% in the horse population, the human disease remains a rare occurrence. With successful transmission, however, a few germs are considered sufficient to trigger a clinical disease.
It traditionally occurs as an occasional (sporadic) single disease in population groups that frequently have close contact with horses or come into contact with infectious material. The risk group therefore includes veterinarians , laboratory and slaughterhouse personnel, animal keepers and other horse-related professions. In areas where snot is not endemic or where it is introduced for the first time (1876 in Cuba ), acute courses of snot in humans occur more frequently. While German soldiers and veterinarians became seriously and acutely ill in World War I , Russians prisoners of war, on the other hand, often showed a chronic course with a good healing tendency. The existence of undetected, mild or symptom-poor (subclinical) infections must therefore also be taken into account. Studies of human autopsies in endemic areas have found snot-associated nodules in many people who have had contact with horses.
In laboratory work, on the other hand, B. mallei is highly infectious, especially when mixtures of gas or air with pathogen particles (aerosols) are formed. Here the disease rate can rise to 46%.
Pathogenesis
Virulence factors
Little is known about the virulence factors . The bacterium sometimes occurs intracellularly , which makes it difficult to combat it by the immune system. Its capsule-like substance offers it another protection against elimination by scavenger cells ( phagocytosis ). Since the bacterium becomes harmless (avirulent) for laboratory mice in an infection attempt after this pseudocapsule has been destroyed, this seems to be a main factor for its virulence.
The disease-causing effect is mainly based on the production of bacterial toxins, which include endotoxins and heat-labile toxins. The genetic material ( genome ) of Burkholderia mallei is at times highly variable . This not only leads to the formation of strains of different aggressiveness, but also to the formation of different surface structures that are no longer recognized by the immune system ( antigenic drift ).
Spread in the host organism
The spread of the pathogen in the host is comparable to that of tuberculosis . As a rule, the nasopharynx represents the entry point ("development of the primary effect"). The subsequent infection of the responsible regional lymph node ("formation of the primary complex"), in which the first snot granulomas are located, occurs via the draining lymphatic pathways ( lymphogenic ) form. Via the bloodstream ( hematogenous ) or lymphogenically, depending on the immune system of the organism, it spreads to other organs, especially the lungs . This is where the snot changes typical of the infection develop. The infection can come to a standstill at this point, or the pathogen is slowly progressing (protracted) washing away ( dissemination ) into other organs and tissues. If the pathogen reaches the cavity of the air ducts after infection of the bronchi and bronchioles , a secondary contact infection of the upper respiratory tract can occur by coughing up the infectious material ( canalicular spread). When the pathogen is absorbed through inhalation, the primary nasal redness occurs , after entry via injured skin, the primary redness of the skin .
immunology
Even surviving an infection does not lead to resilient immunity , that is, the organism is not protected from a new disease . As part of the immune reaction, the host produces agglutinins to immobilize the pathogen, which can be detected in serological diagnostics. Despite the formation of antibodies, the actual effective immune response is mediated by macrophages and T cells . The administration of inactivated vaccines occasionally led to a reduction in susceptibility to the infection in individual animals, but does not offer any generally reliable protection. In the past, boosting the immune system by administering Mallein could in individual cases lead to a clinical improvement in snot-sick horses. New research results show in animal experiments an improved protective effect of the organism through administration of γ-interferon -stimulating substances. These so-called cationic liposome DNA complexes (CLDC) could serve as the basis for the development of exposure prophylaxis .
Clinical picture
Symptoms and course in horses
The snot disease in horses can be acute or chronic; latent infections are also possible. Depending on where the changes manifest, a distinction is made between the following types of snot:
- Malleus farciminosus (red skin)
- Malleus humidus (red nose)
- Malleus pneumonia (lung disease)
This classification is historical and is no longer relevant today. The transitions between the individual forms are fluid, and the animals often show several symptom complexes at the same time. The simultaneous occurrence of all forms was previously also known as red worm disease . The incubation period varies between three days and several weeks.
Acute snot
The acute form is typical for donkeys and crossbreeds and is also observed in around three to twelve percent of horses suffering from snot. The acute snot begins unspecifically with chills and high fever (40–41 ° C), unilateral or bilateral nasal discharge and reddening ( hyperemia ) of the head mucous membranes.
The pharyngeal canal lymph nodes as well as neighboring lymph vessels and lymph glands are often swollen, painful and sometimes riddled with abscesses . On the mucous membranes of the nose, sinuses, trachea , larynx , pharynx and the skin, large areas of liquid-ulcerated ( diphtheroid ) plaque, nodules and individual ulcers form , which spread rapidly. The palm-sized areas of the skin can die off ( skin gangrene ). The connective tissue layers ( submucosa ) of the head mucous membranes, the subcutaneous tissue and the muscles are sultry, soaked with bloody fluid (inflammatory exudation) and swollen. The accumulation of tissue water ( edema ) in the nasal mucous membrane and in the larynx area together with the viscous nasal secretions make breathing difficult. In the course of the disease, the nasal discharge changes its appearance from watery to slimy-purulent to bloody-oozing. It can be mixed with food or saliva. The painful involvement of the head of the pharynx leads to regurgitation of food and difficulty swallowing, so that food consumption decreases quickly. Occasionally, purulent discharge from the eye develops, and in individual cases central nervous symptoms are observed. The donkey has described a peculiar stiff neck and head posture as well as lameness.
The animals also show reluctance to move, emaciation , diarrhea and loss of protein via the kidneys ( proteinuria ). They quickly become obsolete and often perish due to pneumonia and multiple organ metastases. Donkeys and hybrids usually develop acute to subacute redness of the nose and lungs, a stormy septicemic course is also possible.
Chronic snot
The chronic snot occurs predominantly in horses. The symptoms are sometimes only weak and can therefore easily be overlooked at the beginning and with little stress. The disease often lasts for years and runs in irregular feverish bouts . The pharyngeal lymph nodes are usually reactively changed (coarse and nodular), but painless and no longer freely movable due to adhesions. Since the lungs are usually involved in chronic events, coughing and breathing difficulties occur. These can vary from only slight dyspnea during exertion to drowsiness. Quick fatigue, emaciation and shaggy fur are frequently observed general symptoms.
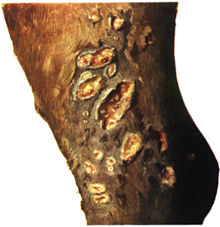
In addition, there may be signs of redness in the nose, which can manifest itself in unilateral or bilateral nasal discharge or nosebleeds. Snotty nodules or ulcers may be absent or appear very late; they are found on the nasal septum and the turbinates as well as on the larynx and in the windpipe. The nasal discharge is gray-white-slimy to gray-yellow-sticky, it can contain blood and scraps of mucous membrane. Healed changes from earlier snot attacks are usually to be found in the form of the typical ice flower- shaped " snot scars " in the nasal mucosa and in the windpipe. Skin redness only occurs in ten percent of cases. Frequently affected areas ( predilection points ) are the areas of increased mechanical stress (harness position, saddle position) on the head, lower abdomen, chest and hind limbs. In the skin and subcutaneous tissue, pea- to walnut-sized nodules and abscesses develop, which disintegrate into ulcers ( ulcerative dermatitis malleosa) , but can also recede again (“flying snot”). Gel-like yellowish-gray pus (farcy oil) drains from opened foci .
The regional lymph nodes swell painfully. If the thigh lymph nodes are affected, the animals show periodic lameness. The lymphatic vessels in the area where the skin changes are drawn in are hardened, twisted and protruding like worms (farcy pipes) . In its further course, pearl-like, abscessing swellings can form ( rosary beads ), some of which drain outwards. The red skin often heals poorly and with excessive scarring . Fist-sized swellings often form in the deeper muscle layers, spread over the trunk. In the advanced stage, swelling of the abdomen, chest, legs and joints occurs. In the area of the hind limbs, the so-called snot phlegmon (dermatitis malleosa diffusa phlegmonasa) , which in rare cases is accompanied by elephantiasis (elephantiasis malleosa) , may develop in the area of the hind limbs due to continued lymph drainage disruption and inflammation of the subcutis . Male animals show inflammation of the testicles ( orchitis ) as well as swelling of the scrotum and external genitals. Snot in the bones leads to increased bone fragility, especially the ribs.
Latent infection
The latent form occurs in horses with a good defensive position. It is usually silent or subclinical (without clinical symptoms), occasionally slight nasal discharge and subclinical orchitis can be observed. As long as there is an equilibrium of forces between host and pathogen, the infection rests. However, when the immune system is weakened, it is activated, so latently infected animals can become excretors again.
Symptoms and course in humans
| Progressive form | Lethality untreated | Lethality Treated |
|---|---|---|
| Septicemia | > 95% | > 50% |
| Lung shape | 90-95% | ≈ 40% |
| Acute local infection | Change of form | ≈ 20% |
| Chronic form | unknown | ≈ 50% |
The manifestations of the disease in humans depend on the entry point and the tendency of the pathogen to spread. A distinction is made between septicemia , pulmonary redness , the acute local form and chronic snot . All forms of progression can merge into one another, whereby various symptom complexes are superimposed. The incubation period depends on the form of manifestation. It is one to five days for the septicemic course and the local form and 10-14 days for the lung form.
The onset of septicemia is marked by acute fever, chills , muscle, head and chest pain . In addition, hot flashes, contact skin rashes , changes in the lymph nodes, bluish mucous membranes ( cyanosis ), photophobia , and granulomatous or necrotizing skin lesions on. Increased heart rate ( tachycardia ), spleen and liver swelling, jaundice and diarrhea indicate the involvement of internal organs. Death usually occurs 24–48 hours after the onset of symptoms from multiple organ failure .
The localized form is characterized by the appearance of nodules, abscesses and ulcers in the mucous membranes, the skin, the lymph vessels or the connective tissue locally at the portal of entry. If the mucous membranes are involved, a mucous-purulent discharge can develop, some of which contains blood. The appearance of snot-specific lesions is accompanied by general symptoms such as fever, sweating and malaise. The regional lymph nodes swell and form abscesses that break open. After one to four weeks of illness, there is a risk of a spreading ( disseminated ) infection, which manifests itself as rashes and abscesses in internal organs. The liver, spleen and lungs are primarily affected, but in principle all soft tissue can be infected. Disseminated infections are often the precursor to septicemia.
The pulmonary form often develops after inhalation of the pathogen or by scattering in the blood stream, from other forms. It is characterized by lung abscesses, pleural effusions, and pneumonia. Symptoms include fever, sweats, cough, chest pain, and shortness of breath. Ulcers and nodules appear in the nose, accompanied by mucous-purulent (mucopurulent) discharge. After a few months, skin abscesses sometimes develop. If left untreated, the lung shape often leads to a septicemic course.
The chronic glanders of man is characterized by nodules, abscesses and ulcers in a variety of organs and tissues. Skin, subcutaneous tissue , liver, spleen, gastrointestinal tract, respiratory tract and the skeletal muscles can be affected. The symptoms are milder than in the acute course, there are periodic febrile recurrences . Weight loss and changes in the lymph nodes ( lymphadenopathy ) are often seen.
forecast
The prognosis depends on the host species, dose of infection, route of infection , virulence of the pathogen, place of manifestation of the disease, course and start of therapy.
Acute snot usually ends fatally after two weeks in humans, equidae, and carnivores if left untreated. Clinically manifest chronic snot can drag on for up to seven years in horses, and over 25 years in humans. Progressive loss of strength will ultimately lead to death by exhaustion (Rotzkachexie), death occurs sometimes suddenly pulmonary hemorrhage one. Chronic reddening of the skin is prognostically unfavorable. With a milder course of the chronic disease, good care and sufficient rest, an almost complete disappearance of the symptoms ( remission ) within three to six months has been observed in horses in the past . Stress factors can lead to a relapse ( relapse ) at any time with a worsening of the clinical picture, but healing is often permanent.
There are experiences from the First World War of the successful recovery of chronically ill people. Latent infections can heal spontaneously or turn into a clinically visible form of the disease.
pathology
Pathological-anatomical changes
The pathological picture varies depending on the place of manifestation, form and age of the disease. While the acute form is more characterized by processes associated with tissue breakdown and the formation of inflammatory secretions ("exudative-necrotizing"), in chronic courses the formation of granulomas and new tissue ("productive-proliferative") dominates. The detection of the specific snot nodules and their various stages of development is of central diagnostic importance ( pathognomonic ).

In acute snot, erosions and snot nodules of variable size (the size of millet grain to the size of a pinhead) are found in the upper respiratory tract, the lungs, skin and lymph nodes, but less often in the liver, spleen, testes and bones. In chronic snot, they can be found in all organs, soft tissues and bones. The color is gray-white-reddish and glassy, the consistency varies from soft to firm. As a sign of chronic infections and healing, there may be central casings or calcium deposits . Outwardly, the nodules are clearly demarcated by a reddened ( hemorrhagic ) border. They often disintegrate, releasing discolored yellowish green pus and forming a wall-like edge. The resulting snot sores can flow together over a large area and spread to cartilage and bone tissue, especially in the mucous membranes of the head. Depending on the tissue layers affected, wound healing takes place without visible scars, through the formation of small white nodules or in the form of the typical snot scars . These are star-shaped or ice-flower-like and have a radiant course of the scar ridges. The nasal septum , turbinates and palate may be perforated .
The pathological picture of the lungs is characterized by enlarged lymph nodes and nodules of variable number and distribution (nodule redness ) . A catarrhal form of pneumonia is often observed in acute snot . This is characterized by the occurrence of lentil- to pea-sized herds that are brown-red solidified and show central softening. Adjacent tissue is soaked in yellowish-sultry, and effusions occur in the pleural cavities .
When several nodules converge, hazelnut to walnut-sized snot nodules develop in the lung tissue . As a result of melting down, caverns are formed . The snot nodules serve, among other things, as the starting point for the so-called lobar malleous pneumonia . This catarrhal-purulent form of pneumonia does not have any peculiarities typical of the pathogen. It can turn into chronic indurative pneumonia , for which the parallel presence of fresh changes in addition to already hardened, cased or connective tissue delineated foci is typical. The active herd contained therein can harbor the pathogen for a long time. The healing of the snot occurs through calcification and continuous formation with coarse connective tissue (callous redness) .
In the skin, subcutaneous tissue and muscles, there are snot nodules with a central, purulent softening, which are partly separated from the environment by connective tissue. The resulting ulcers have an opening the size of five pennies (approx. 18 mm in diameter), red, greasy ulcer base and a jagged, raised edge (boiler ulcers ) . The walls of the lymphatic ducts and their surroundings are thick and greasy. The lymph nodes are enlarged, greasy, interspersed with snot nodules and grown together with their surroundings through connective tissue growth. Snotty lymphangitis on the limbs is often associated with extensive inflammation of the subcutaneous tissue . In this context, massive, diffuse connective tissue formations with nodular thickening of the skin can also be observed ( pachydermia ) .
histology
The typical snot nodules represent specific snot granulomas from a histological point of view. The center of these granulomas contains a zone of cell death ( necrosis ), which is characterized by the presence of cell debris, neutrophilic granulocytes and bacteria. In the subacute phase of the disease, this focus is sealed off from the outside by the epithelial cells ( histiocytic demarcation ). The next layer on the outside contains a mixture of different immune cells: lymphocytes , plasma cells and macrophages . The zone of fiber-forming cells ( fibroblasts , fibrocytes) delimits the granuloma from the outside; as the disease progresses, this layer is increasingly reinforced by the storage of collagen fibrils.
Differential diagnoses
In the horse, in addition to the are strangles the virally induced rhinopneumonitis , the Rhodococcus equi infection (Rhodokokkose) and other pneumonias bacterial origin that Luftsackempyem , Lungenparasitosen, Pseudorotz ( melioidosis ), tuberculosis, pseudotuberculosis , lymphangitis epizootica , sporotrichosis , dermatophilosis and dermatomycoses exclude . In humans, pseudorotis, typhus , syphilis , tuberculosis, rotlauf , lymphangitis and pyaemia are the most common types of differential diagnosis.
Diagnostic procedures
Diagnostics on animals
A reliable diagnosis based on external changes is usually not possible due to the highly variable clinical picture. Above all, the clear demarcation from the gland causes difficulties. A suspected clinical diagnosis can, however, be made if there are visible changes in the nasal region typical of red blood or if the individual symptom complexes occur simultaneously. The preliminary report is of great importance. In the past, attempts were made to substantiate suspicions of snot through unsafe methods such as opening the maxillary sinuses , peeling off the pharyngeal lymph nodes or puncturing the lungs. To provoke a clearer clinical picture, the animals were exposed to increased stress or given mallein.
Imaging procedures ( X-rays ) can provide further information in the case of red lungs and involvement of internal organs or bones . Snot can also be diagnosed pathologically-anatomically or histologically by detecting the granulomas that are typical of snot .
Direct pathogen detection
Conventional laboratory methods
Sputum and pus from closed nodules or fresh injuries to the skin and nasal mucosa are particularly suitable for the detection of pathogens . Straight or slightly curved rods with rounded ends, approx. 2.5 µm × (0.3 ... 0.8 µm) in size, are visible under the microscope . The predominantly extracellular rods are only weakly or irregularly stained in the Gram stain . The microscopic detection from tissue sections is difficult; immunohistochemical methods are used here. The cultural cultivation is preferably carried out in complex nutrient media with the addition of glycerol , horse serum and antibiotics. After 48 hours of incubation at 37 ° C., a moist, creamy-white coating becomes visible, which smells of hops and becomes darker and tougher with further incubation (similar to honey drops). The final determination of the species includes, in addition to the proof of immobility, also the biochemical characterization.
Diagnostic animal experiment
In addition, animal experiments can be used for direct pathogen detection . In the past it was primarily used for the reproduction and cultivation of the snot bacterium. Traditionally, guinea pigs, mice, hedgehogs and cats are suitable as vaccines . After subcutaneous or intraperitoneal infection, male guinea pigs develop fatal testicular and peritoneal inflammation within a few days (Strauss reaction) .
Molecular biological detection methods
The molecular genetic detection of the pathogen and the clear differentiation from B. pseudomallei is now also possible through a specific PCR , which is, however, still in the test phase ( validation ). Other molecular genetic methods such as RFLP , pulsed field gel electrophoresis , 16sRNA - DNA sequencing and VNTR are not available in all laboratories and tend to be used for epidemiological questions.
Indirect pathogen detection
Various serological methods or allergic reactions are used for indirect pathogen detection, depending on the issue . The problem with all of these detection methods is, on the one hand, the inability to differentiate from the causative agent of the pseudorot, which can lead to false positive results due to cross-reactions . Since each method detects different points in time within the course of the disease, false negative results are possible when using a certain test alone.
Serological detection methods
The Serumlangsamagglutination (SLA) is only suitable for the detection of acute illnesses from the sixth day after infection. ten to twelve weeks after infection, it is already negative again. The complement fixation reaction (KBR) becomes positive from the second week of infection and detects chronic snot in horses. The appearance of specific complement- binding antibodies ( seroconversion ) is sometimes delayed (fourth week after infection) and in individual cases may not occur at all. However, due to its overall high sensitivity (90–95%) and specificity (99%), the method is prescribed for controls in international animal traffic. The test is not suitable for donkeys, hybrids and pregnant mares. Reliable seroconversion does not occur in humans. The combined and repeated use of agglutination and complement fixation reactions led in many places to the successful eradication of snot and is still relevant today.
Other methods that are used occasionally are the Rose Bengal test (Russia), precipitation tests, the hemagglutination inhibition test and immunofluorescence . Various ELISA methods are used in snot diagnostics and have a sensitivity comparable to that of KBR. New techniques based on monoclonal antibodies also allow differentiation between B. mallei and B. pseudomallei , but have not been validated. Immunoblot methods are now used to confirm positive or questionable KBR results.
Painting
The Mallein test is based on a late- type allergic reaction mediated by T cells . The mallein is a purified , soluble component from the cell wall of the snot pathogen. Since its discovery in 1890, Mallein has been used extensively in the eradication of snot. A distinction is made between three test forms:
- For the Mallein eye test , a few drops are placed in the conjunctival sac ; a positive reaction is expressed within 24 hours by purulent eye discharge and swelling of the eyelids and the facial area.
- In the case of intradermal-palpebral inoculation , 0.1 ml Mallein is injected into the skin of the lower eyelid, reagents show swelling of the eyelids and purulent eye discharge after 24–48 h.
- In the subcutaneous test , 2.5 ml Mallein is injected into the neck area; snot-sick animals show sharply delineated, painful swellings at the inoculation site after 24–48 h .
In all three procedures, a positive reaction is usually accompanied by a high fever with a typical course and disorders of the general well-being.
The subcutaneous method is the oldest, but has a significantly poorer detection power compared to the others. As the most reliable test, the intrapalpebral form was used by England, France, Italy and Romania to cleanse snot from the First World War. However, the Mallein eye test has established itself over the injection forms due to its technical and hygienic advantages. The Mallein test also records inactive stages of the infection. Although it is considered to be the most sensitive procedure overall, it comes to false negative results in donkeys and hybrids (only positive from the third week after infection) as well as in the chronic snot of horses in an advanced stage ( anergy ). For this reason, and also because of the interaction (interference) of the Mallein test with subsequently carried out serological tests ( KBR ), this method is no longer used throughout the EU .
therapy
Historical attempts at therapy
Although the use of numerous therapeutic agents against snot was documented, especially in the middle of the 19th century (e.g. snot sulfur, halogen compounds , phenol (outdated carbolic acid )), the futility of sustainable attempts at healing was recognized early on. In later times the use of snot vaccines and the administration of Mallein did not lead to the desired safe success in humans and animals. Until well into the 20th century, snot was therefore generally considered incurable. Successes could only be observed in isolated cases in the case of chemical burns or cauterization of locally limited changes. The veterinary therapy of snot-sick horses was also economically unprofitable and was critically questioned due to the danger to humans and animals. The main focus of control in animals was therefore on prophylactic measures such as improving hygiene , strengthening general resistance and killing clinically ill animals.
Current therapy
In countries where the snot has been successfully eradicated, there is an absolute therapy ban. In endemic areas without adequate compensation payments for eradicated animals, antibiotic therapy attempts represent an alternative to killing for economic reasons. In humans, snot is treated with the help of antibiotics . Since most cases of human snot disease fall into the pre-antibiotic era, the available data on effective therapy is limited. The pathogen is already naturally resistant to many classes of antibiotic active ingredients . The choice of possible therapeutic agents is further restricted by the reduced penetration capacity of active ingredients into the interior of the snot changes, but also by the partially intracellular occurrence of the pathogen. Doxycycline , rifampicin and ciprofloxacin were found to be effective under laboratory conditions ( in vitro ) . An effective effect for sulfadiazine has been demonstrated experimentally in living animals ( in vivo ) . In the recent past, the success of therapy in laboratory infections was due to sulfadiazine in 3/4 of the cases; Aureomycin and doxycycline were also found to be effective. Therapy recommendations vary depending on the severity and location of the disease, but usually several antibiotics are administered over a period of at least two months. Drug therapy can take up to a year; in severe cases, it is supported by surgical interventions. It was recommended (as of 2009) for antimicrobial therapy in humans initially the intravenous administration of imipenem and doxycycline for two weeks and then doxycycline orally for six months.
Combating snot
Prevention in humans
There is no vaccination . Post- exposure antibiotic prophylaxis may be indicated in some cases . The targeted work with the red pathogen and infectious materials is limited to laboratories of biosafety level 3 ( protection level 3) and higher. Particular emphasis is placed on measures to prevent the release of pathogens and spread through the air. The key points are spatial separation from other laboratory areas, strict access control, locks, work under safety workbenches , negative pressure in the work area and filtering the exhaust air. Strict protective measures must also be taken when handling infected animals, sick people or contaminated materials. The necessary protective clothing includes gloves and a face shield. Additional measures to protect against aerosols may be indicated. Although human-to-human transmission is rare, patients with snot should be isolated . The best prevention consists in the eradication of the red germ from the animal herds.
Reporting requirement
In Austria, suspected cases of illness and deaths from snot are notifiable in accordance with Section 1 (1) number 1 of the 1950 Epidemic Act . Doctors and laboratories, among others, are obliged to report this ( Section 3 Epidemics Act).
Animal health regulations
Since snot can be reintroduced into disease-free areas at any time due to the occurrence of endemic herds, the ban on the importation of solipeds from contaminated regions as well as the diagnostic monitoring of international animal traffic are important main goals of the control of snot. Legal bases are § 12 of the Animal Disease Act , the collection of methods of the FLI , the internal market animal disease protection regulation , the directives 90/426 / EEC, 90/425 / EEC and 2009/156 EC as well as the Manual of Standards for Diagnostic Tests and Vaccines .
Measures in the event of an outbreak
According to the Regulation on notifiable animal diseases is the snot obligation . The notification must be made immediately to the responsible veterinary office, even if there is a mere clinical suspicion . It entails an immediate ban on the population by the official veterinarian as well as extensive epidemiological and diagnostic follow-up examinations of all contact animals. If an outbreak is detected, any therapy is prohibited - all sick and infected animals must be killed without blood withdrawal and the carcasses disposed of . The basis for a uniform procedure up to April 2011 was the guidelines introduced in 1974 for the detection of snot (malleus) in solipeds by means of serological and allergological test methods, after which the infection was determined by means of blood serological tests (KBR, LA) and additionally the Mallein sample. Since false-positive results are possible with these methods, the official collection of methods of the Friedrich-Loeffler-Institut (instructions for laboratory diagnostics of notifiable animal diseases) was adapted in April 2011 . If there is a suspicion of snot, blood samples must be taken every two to three weeks and examined using a KBR. Positive results must be verified immediately with a Western blot . Then a cultural detection and a PCR for the molecular biological detection of the pathogen are carried out.
Suspected contagious animals are to be subjected to a six-month disease prophylactic observation while maintaining the herd ban. During this period, blood samples are taken every two to three weeks and examined using a KBR. If no further suspicious animals are found during these follow-up examinations, two further blood samples are to be taken two weeks after the last normal test. In parallel, the prescribed intermediate disinfection took place, including the stables and all potentially contaminated objects. Dung, litter or feed residues are to be burned. Alternatively, they can be disinfected, stacked and plowed under after three weeks. After the epidemic has subsided and the final disinfection has been carried out, the stock lock will be lifted.
Measures to protect free territories
For international animal traffic with third countries, the respective import regulations of the destination country apply. The Internal Market Animal Disease Protection Ordinance is relevant for the import of equidae into Germany . It transposes Directives 90/426 / EEC and 90/425 / EEC, which regulate imports from third countries and the intra-community movement of animals and goods according to community-defined requirements. Commission Decision 93/197 / EEC lays down the animal health conditions and the authentication method for the import of registered equidae as well as equidae for breeding and production. According to this, a check for the absence of snot antibodies by means of a complement fixation reaction is required for imports from certain third countries mentioned by name . This must take place during a 30-day quarantine under veterinary supervision in the country of origin. No snot may have appeared in the herd of origin or within a 30 km radius in the past twelve months. Forage and bedding intended for transport must be free from pathogens. Animals destined for permanent residence are subjected to a further four-week quarantine in the country of destination.
The fact that no single allergological or serological test can offer a hundred percent certainty has always been a problem . Repeatedly false positive results occur in the KBR with high sample volumes, which require further diagnostic clarification. However, latently infected animals pose a particular risk to disease-free areas, which give false negative results due to a lack of or delayed antibody formation in the KBR . This was last the case in 2005 with a sport horse imported from Brazil , in which the disease broke out due to the stress of transport, climate change and changes in feed.
Meat hygiene
The slaughter permit is to be refused for snot-sick or suspicious animals. The skinning of carcasses is also prohibited . As part of the meat inspection , a systematic examination of the skull and respiratory tract for changes in snot is to be carried out in solipeds. If the result is positive, the animal's body is deemed unfit for human consumption and may not be used as animal feed .
History of snot
Ancient and Middle Ages

Already Aristotle mentions in his Historia animalium (Volume VIII, Ch. 25) has an ass occurring, fatal disease. The described symptoms and course clearly indicate the acute form of snot disease typical for this species. Also in the Corpus Hippiatricorum Graecorum , an early medieval compilation based on hippiatric works of the 3rd – 5th centuries. Century, the snot disease is mentioned.
Ancient writings do not always allow the described clinical pictures to be clearly assigned to snot. In Book III of Mulomedicina Chironis (late 4th century), several symptoms are presented under the term morbus malleus , for which, in addition to various manifestations of snot in antiquity and the Middle Ages, other, in some cases chronically incurable, horse diseases come into question. What is remarkable at this point is the knowledge about the possible transferability of snot through “spoiled air”. In the 5th century AD, the Roman military historian Vegetius Renatus took up parts of the Mulomedicina Chironis again for his Ars veterinarius sive Mulomedicina and referred to the snot disease as malleus .
In the Middle Ages the snot belonged to Germanic law to guarantee defects , and was under the names Hauptmönigkeit , Hauptmörigkeit , Hauptsichtig , hood table , Mordisch and Atticum Profluvium known.
17th and 18th centuries
It was not until the 17th century that there were sparse written references to snot disease. The renaissance author Filippo Scacco described the redness of the skin in his Trattata di Mescalzia in 1603. The stable master Jacques Labessie de Solleysel mentions the disease in his work Le Parfait Mareschal 1664 and in it draws attention to the risk of infection: "It (the poison) infects the air and takes hold of all horses that are under the same roof" . The fact that the horse disease with its manifestations "snot" and "worm" was known and quite common is indicated by its assignment to the warranty deficiencies in animal purchase law. From around 1780 the first bans on the slaughter of snot-sick horses for human consumption also existed.
Due to its increasing economic and military importance, snot disease moved into the focus of scientific interest in the 18th century. To research and combat snot and rinderpest , the first veterinary educational institution in Europe was founded in Lyon in 1762 (cf. Claude Bourgelat ). In 1771, the first authoritarian measures to curb snot disease were enacted in German-speaking territory.
Erik Nissen Viborg recognized in 1795 that "snot" and "worm" represent only two forms of the same disease, and was able to prove their contagious character for the first time in 1797 by inoculating infectious material and by combining healthy and sick horses. However , at the turn of the century, unsuccessful infection attempts by the Alfort School of Veterinary Medicine ( École nationale vétérinaire d'Alfort ) led to the fact that the transferability was again doubted by well-known contemporary professors and doctors. The resulting relaxation of quarantine and control measures that had already been introduced resulted in a rapid and strong spread of the epidemic in many places, but particularly in France.
19th century
The snot disease caused difficulties in recognizing epidemiological connections due to its highly variable clinical picture and the existence of silent, latent courses of infection. It was often mistaken for other infections, and the demarcation from the gland caused particular problems . While the so-called contagionists to a transmissible agent (Rotzcontagium) believed their opponents championed the theory of spontaneous generation ( spontaneous genesis ) . Bad husbandry conditions, among other things, were blamed as triggering factors. In addition, until the beginning of the 20th century, the erroneous opinion persisted that snot could arise from other horse diseases.
Early control measures in the 19th century were based on the obligation to report snot disease, informing the public in the event of an outbreak of disease, killing orders and compensation for animals declared by a veterinarian to be sick with snot, quarantine and possible killing of contact horses and thorough cleaning of contaminated stables.
The first clear case of snot disease in humans was published in 1821, but the syndrome had been known for a long time from those researchers who had intensive contact with snot-sick horses. In 1837 and 1838, Rayer and Leblanc again demonstrated the transferability of acute snot infections to the human organism and showed that pathological changes in humans and animals corresponded. It was not until 1849 that the professors Francois Saint-Cyr and Eloy Barthelemy from the Lyon school succeeded in proving that chronic snot was transferable.
Otto Bollinger (1868) and Andreas Christian Gerlach (1874) independently demonstrated the exclusive transferability of the disease through contact with sick animals or their excretions. In 1881, the red pathogen was first detected microscopically in organ material by Victor Babes. It was not until 1882 that Charles-Joseph Bouchard , Friedrich Loeffler and Wilhelm Schütz successfully cultivated it . In 1886 the course of the infection could be satisfactorily clarified (fulfillment of the Koch-Henle postulates ).
Developed vaccines and therapeutics have shown no success in the fight against the red pathogen. After the sole eradication of snot-sick animals did not lead to a decrease in the epidemic, the importance of clinically inconspicuous carrier animals was recognized for the first time. Confirmation of a clinical suspicion of snot at this point in time was only possible through the uncertain experimental infection of test animals.
The discovery of the Mallein in 1890 by Christophor Ivanovitsch Hellman (Russia), Otto Ivanovitsch Kalning ( Estonia ) and Leonard Pearson (USA) also made it possible for the first time to identify latently infected carriers and represented a first important step on the way to eradicating the pathogen The agglutination reaction of red blood pathogens with the serum of snot-sick horses, observed by the bacteriologist John MacFadyean , formed the basis for the first serological method in snot diagnosis, serum slow agglutination (SLA).
Since horses were still the most important means of transport, the disease spread to a new extent in the 19th century through wars, settlers and the increasing international animal traffic, especially by sea. At the end of the century, economically significant horse losses as well as an increasing awareness of the threat to humans led to the establishment of standardized state control programs in many countries. For example, snot control was included in the Canadian Animal Contagious Diseases Act in 1897 , and the Glanders and Farcy Act came into force in India in 1899 .
20th century
After the development of the Mallein test as a diagnostic method, the killing and compensation of positive animals became compulsory in many countries. Nevertheless, the success initially failed because contact animals were withdrawn from testing in some places or, just like positive, inconspicuous horses, were quickly sold to uninvolved third parties in order to avoid financial losses. Particularly on the Canadian border with the USA, full import tests were not always possible due to uncontrolled settler flows and consequent tracking of contact animals was almost impossible. Only after adequate compensation was extended to include the killing of contact animals, the existence of a clinically inconspicuous carrier status was generally accepted, and the undermining of the killing order became a criminal offense, the eradication of snot made rapid progress worldwide. Control efforts, however, were lengthy and costly, with Canada paying more than $ 1 million in compensation for over 13,000 animals killed from 1904 to 1938. In the years 1876–1886 the animal losses due to snot amounted to 20,500 animals for Prussia alone, which were compensated with 4.5 million marks . The first European countries to successfully eradicate B. mallei were Northern Ireland (1919), Ireland (1920), and Denmark and Great Britain (1928).
In Germany, the uniform state control of snot by the veterinary police had already started with the Reichsviehseuchengesetz of 1880, which was further improved by subsequent laws in 1894 and 1909. After the mandatory meat inspection was imported in 1903, dozens of cases of snot in horses were uncovered during slaughter, thus eliminating other sources of disease. In the years 1886–1902 a total of 13,000 animals were officially reported to be sick with snot in Germany. West Prussia, Poznan and Silesia (especially Russian border areas through cattle smuggling) as well as individual regions in Lorraine, Upper Bavaria and Württemberg were particularly heavily contaminated.
With the introduction of the complement fixation reaction, snot diagnostics were refined in such a way that, with a seroprevalence of 0.3%, snot in German army horses at the beginning of the First World War hardly played a role. The renewed spread of the epidemic during the war of movement was mainly due to contact with snot-sick Russian prey horses or contaminated stables, but also to the lack of control over replacement horses ( remonts ). In the following trench warfare succeeded by that of Robert von Ostertag excited establishment of blood investigative bodies back the Rotzerkrankung for Army horses and also in the horse population of the civilian hinterland. From August 1914 to the end of the war in 1918, more than 15.5 million blood samples were serologically examined and a total of around 31,000 horses were killed for snot in the German field army. Despite the war, the importance of snot in Germany continued to decline, so that in 1929, with the existence of a single last epidemic farm, the final eradication was within reach. In the Reichswehr epidemic regulation of 1930, however, snot was still notifiable. After the last peak of the epidemic in World War II , during which the disease no longer achieved its former importance, it has been considered eradicated in Germany since the mid-1950s.
In the Russian Empire , due to the political and agricultural conditions and the lack of veterinarians, snot had gained ground. It was not until 1903 that an Animal Disease Act was introduced, which, however, was not widely used and limited the control of snot to the Merzen of clinically ill animals. 1908–1912 approximately 117,000 animals with clinical symptoms were killed on Russian territory. The Ukraine , Crimea , the Volga region and the Caucasus were particularly heavily contaminated . The First World War and the civil war that followed drove the spread of snot further, so that the Russian horse population was seriously endangered in the 1920s. The proportion of Mallein reagents between 1925 and 1929 was between 0.6 and 38.9%, depending on the area, the acutely snotty animals usually made up about 1/10 of this. In 1925 a law to combat snot was passed, which took into account the widespread spread of the disease in the country and was economically viable. However, the simple and robust concept was only successfully implemented in 1929 after the forced expropriation and concentration of animal herds in sovkhozes, collective farms and transport companies. The rehabilitation program was based on the introduction of animal identification as well as planned clinical, allergological and serological controls. Sick or suspect animals with equivocal test results were culled . Animals that only reacted to the Mallein and showed no clinical symptoms had an unclear excretory status. They were therefore kept under quarantine in specially selected, remote homesteads for further use. At regular intervals, these mallein economies were checked clinically, allergologically and serologically so that animals with active infection could gradually be killed. This concept led to the rehabilitation of entire stretches of land. In the mid-1950s, all horses in the Soviet Army were also considered to be free of snot.
The Second World War delayed the successful eradication of snot in many countries, but by 1965 the disease had been reduced in most nations around the world. After three major outbreaks among army animals in the 1970s, snot was also considered eradicated in India in 1988.
Snot as a biological weapon
Since the pathogen is considered to be easy to distribute and the snot disease has high morbidity rates and medium mortality rates, B. mallei was classified as a category B biological warfare agent and handling of the pathogen was restricted to level 3 safety laboratories.
Various circumstances predestine the red pathogen to be the ideal biological tool (agent) . The disease has become unknown in the West due to the successful eradication, the clinical picture of the zoonosis is variable, the first symptoms are non-specific. For this reason, once exposure has taken place, the diagnosis and initiation of therapy could be delayed even in areas with advanced medical care, leading to increases in morbidity and mortality as well as mortality. Although snot is curable, there is little therapeutic experience in humans. In addition, there is a risk of genetically modified strains with increased virulence and atypical antibiotic resistance .
Since the pathogen still occurs in the wild, it is relatively easily available. As diseases in laboratory staff have shown in the past, the infection through inhalation of the pathogen is very likely. The bacteria can easily be brought into aerosol form. The susceptibility of horses and other animals leads to the further spread of the pathogen in an emergency by maintaining heterologous chains of infection .
Effective prevention through active or passive vaccination is not yet possible.
Use of snot against animals
Snot was used as one of the first biological weapons in the 20th century. During the First World War, horses were still of great military importance due to their use in cavalry and artillery units and for transporting materials. The German Reich carried out a series of acts of sabotage against the USA, Russia, Romania and France on both the western and eastern fronts . In addition, German agents such as Anton Dilger attempted to infect horses, mules and cattle, which were intended to supply the Allied troops, with snot cultures in the USA before they were shipped to Europe. Similar steps were taken against war-neutral nations such as Spain and Argentina in order to prevent the delivery of equidae to the enemy troops. In addition, 4,500 mules were infected with snot in Mesopotamia . French cavalry horses were also deliberately infected with snot. By infecting numerous horses and mules on the Eastern Front , Germany and its allies succeeded in obstructing the movement of the artillery troops and the progress of the supply convoys.
Use of snot against people
The targeted use of snot pathogens against people was frowned upon in the First World War. In connection with the rise in the number of cases in animals, an increased incidence of snot disease in humans was observed in the contaminated areas during and after the war . Between 1932 and 1945, Japan developed biological warfare agents based on B. mallei and used them to infect animals, civilians and prisoners of war in the Ping Fan Institute ( unit 731 ) in occupied Manchuria . However, it was never used openly on a battlefield. The former Soviet Union was accused of using snot as a biological weapon against opposition troops in Afghanistan from 1982 to 1984.
Current developments
In response to the suspected threat of biological weapons developed by Japan and Germany, work with biological warfare agents began in the United States in 1942 at Fort Detrick . The red pathogen was researched into its possible uses, but it was not developed as a weapon. In 1972 the United States signed the Bioweapons Agreement and all work on offensive weapons research ceased. However, defense research is still being conducted worldwide. Its aim is to develop effective vaccines and research virulence factors and resistance mechanisms. Attempts to acquire or use snot pathogens by terrorist groups are not yet known.
literature
The cited literature also served as the source for the article.
- Michael Rolle, Anton Mayr (ed.): Medical microbiology, infection and epidemic theory. 8th edition. Enke, Stuttgart 2007, ISBN 978-3-8304-1060-7 .
- Thomas Blaha (Ed.): Applied epizootiology and animal disease control. VEB Gustav Fischer, Jena 1988, ISBN 3-334-00204-7 .
- Olof Dietz (Hrsg.), Bernhard Huskamp (Hrsg.): Handbuch Pferdepraxis. 2nd Edition. Enke, Stuttgart 1999, ISBN 3-432-29262-7 .
- World Organization for Animal Health: Glanders. (PDF; 115 kB) In: Manual of Diagnostic Tests and Vaccines for Terrestrial Animals .
- Bridget Carr Gregory, David M. Waag: Glanders (PDF), in: Medical Aspects of Biological Warfare , Borden Institute
- Center for Food Security & Public Health, Iowa State University: Glanders (PDF; 118 kB)
- Angela von den Driesch , Joris Peters: History of veterinary medicine. 5000 years of veterinary medicine. 2nd Edition. Schattauer, Stuttgart 2003, ISBN 3-7945-2169-2 .
- Franz Friedberger, Eugen Fröhner: The snot. In: Textbook of the special pathology and therapy of domestic animals. Vol. II. Ferdinand Enke, Stuttgart 1904, pp. 437-469.
- E. Lührs: Snot. In: Valentin Strang, David Wirth: Veterinary medicine and animal breeding: An encyclopedia of practical animal science. Volume VIII. Urban and Schwarzenberg, 1930, pp. 641-662.
- Snot worm disease in horses. In: O. Röder (Ed.): Haubner's Landwirtschaftliche Tierheilkunde. 14th edition. Paul Parey, Berlin 1907, pp. 417-426.
- Hermann Mießner: Maleus. In: War animal epidemics and their control. 5th edition. M. & H. Schaper, Hannover 1941, pp. 9-44.
- Robert-Rafael Koch: The fight against the snot of the equines under the conditions of Western and Eastern Europe. Diss. (Med. Vet.), Giessen 1954.
- Karl Wurm, AM Walter: Infectious Diseases. In: Ludwig Heilmeyer (ed.): Textbook of internal medicine. Springer-Verlag, Berlin / Göttingen / Heidelberg 1955; 2nd edition ibid 1961, pp. 9-223, here: pp. 219 f.
Web links
- Glanders and Melioidosis Fact Sheet at the Center for Biosecurity at the University of Pittsburgh Medical Center (UPMC)
- Overview of snot (PDF) in the Manual of Diagnostic Tests and Vaccines for Terrestrial Animals 2008 of the World Organization for Animal Health (PDF; 80 kB)
- Presentation on snot at the Veterinary Faculty of the University of Munich (PDF; 1870 kB)
- Burkholderia mallei Resource Page
Individual evidence
- ^ A b c Heinrich Neubauer: Zoonoses in Germany. An overview of occurring and possible pathogens. In: Deutsches Tierärzteblatt. (Dt. Täbl.) 56, 2008, pp. 1342-1346.
- ↑ MS Redfearn, NJ Palleroni, RY Stanier: A comparative study of Pseudomonas pseudomallei and Bacillus mallei. In: Journal of general microbiology. Volume 43, Number 2, May 1966, pp. 293-313, ISSN 0022-1287 . PMID 5962362 .
- ↑ Wolfgang Pfeifer (Ed.): Etymological Dictionary of German. 2nd Edition. Akademie, Berlin 1993, p. 1140; Kluge: Etymological dictionary of the German language. Arranged by Elmar Seebold. 24th edition. De Gruyter, Berlin / New York 2002, p. 772.
- ↑ See for example: A. Müller, RW Schlecht, Alexander Früh, H. Still The way to health: A faithful and indispensable guide for the healthy and the sick. 2 volumes, (1901; 3rd edition 1906, 9th edition 1921) 31st to 44th edition. CA Weller, Berlin 1929 to 1931, Volume 1 (1931), p. 188 f .: Der Rotz (Wurm; Malleus) .
- ↑ See von den Driesch / Peters, p. 49.
- ↑ See von den Driesch / Peters, p. 30.
- ↑ a b c oie.int
- ^ Marianne Abele-Horn: Antimicrobial Therapy. Decision support for the treatment and prophylaxis of infectious diseases. With the collaboration of Werner Heinz, Hartwig Klinker, Johann Schurz and August Stich, 2nd, revised and expanded edition. Peter Wiehl, Marburg 2009, ISBN 978-3-927219-14-4 , p. 218 f.
- ↑ Animal Disease Report 2011 by the BMELV . In: Deutsches Tierärzteblatt. (DTBL) Volume 60, May 2012, pp. 714–715.
- ↑ FLI detects snot infection in imported horses
- ↑ Veterinary medicine: The snot is back. In: Research News. Deutschlandfunk , January 29, 2015, accessed on January 30, 2015 .
- ↑ "Snot" -specific DNA was detected in skin samples from a killed horse. Lower Saxony Ministry of Agriculture, January 28, 2015, accessed on February 22, 2015 .
- ↑ Infectious disease snot found in horses in Lower Saxony - further investigations started in Schleswig-Holstein. Press release. Ministry for Energy Transition, Agriculture, Environment and Rural Areas in Schleswig-Holstein, January 30, 2015, accessed on June 30, 2015 .
- ↑ Still no suspected cases of snot in horses in Schleswig-Holstein. Press release. Ministry for Energy Transition, Agriculture, Environment and Rural Areas in Schleswig-Holstein, February 4, 2015, accessed on June 30, 2015 .
- ↑ Cf. Gregory & Waag, p. 124.
- ↑ See http://www.indianexpress.com/news/22-horses-detected-with-glanders-in-nainital/31759/
- ↑ See http://www.flutrackers.com/forum/showthread.php?t=28997
- ↑ M. Elschner, E. Liebler-Tenorio, P. Wohlsein, E. Lange, V. Kaden: Rotz - A Rotz case imported to Germany in 2006 shows that this disease is an omnipresent danger ( Memento from December 17th 2010 in the Internet Archive ) (PDF; 7.1 MB) In: Friedrich-Loeffler-Institut. 2006 annual report . Pp. 66-71.
- ↑ a b See World Organization for Animal Health: Glanders (PDF; 115 kB).
- ↑ Cf. Gregory & Waag, p. 127.
- ↑ a b See Friedberger & Fröhner, p. 440.
- ↑ a b See Gregory & Waag, p. 125.
- ↑ E. Yabuuchi, Y. Kosako, H. Oyaizu, I. Yano, H. Hotta, Y. Hashimoto, T. Ezaki, M. Arakawa: Proposal of Burkholderia gen. Nov. and transfer of seven species of the genus Pseudomonas homology group II to the new genus, with the type species Burkholderia cepacia (Palleroni and Holmes 1981) comb. nov. In: Microbiology and immunology. Volume 36, Number 12, 1992, pp. 1251-1275, ISSN 0385-5600 . PMID 1283774 .
- ↑ See Lührs, p. 646.
- ^ History of elephant keeping in Cologne Zoo
- ↑ a b c See Friedberger & Fröhner, p. 441.
- ↑ See Lührs, p. 647.
- ↑ a b See Mießner, p. 10.
- ↑ a b See Mießner, p. 13.
- ↑ a b Haubner, p. 418.
- ↑ Hertwig: Transmission of animal infectious substances to humans. In: Yearbook of domestic and foreign entire medicine. Volume 8, No. 1, 1835, p. 15.
- ↑ a b See Lührs, p. 657.
- ↑ See Lührs, p. 648 f.
- ↑ See Jacob Reiff: Snot at lions and tigers. Diss. (Vet. Med.) Gießen 1919, p. 5 (introduction).
- ↑ a b c d Cf. Lührs, p. 655.
- ^ Cf. Basil Clarke: The Story of the British War-Horse from Prairie to Battlefield In: The Great War - The Standard History of the All Europe Conflict . Volume 9, Amalgamated Press, London 1917.
- ↑ See case report of a neglected Bengali cavalry regiment 1883 . (Army (India) - Veterinary Department - Glanders in Cavalry Regiments. HC Deb 05 March 1883 vol 276 cc1408-9)
- ↑ Cf. Army Medical Services Museum: History of the Royal Army Veterinary Corp ( Memento of the original from August 21, 2008 in the Internet Archive ) Info: The archive link was automatically inserted and not yet checked. Please check the original and archive link according to the instructions and then remove this notice. .
- ↑ a b See The Confederate Cavalry and the "Great Glanders Epizootic" ( Memento of October 7, 2014 in the Internet Archive ).
- ↑ Cf. Paul Lukoschus: The movement of the notifiable equine diseases (snot, mange, Beschälseuche) in the German Reich from 1910 to today and the causes for this movement. Diss. (Med. Vet.) Gießen 1964, p. 23.
- ↑ See Koch, p. 20.
- ↑ See JB Derbyshire: The eradication of glanders in Canada. In: The Canadian veterinary journal. La revue vétérinaire canadienne. Volume 43, Number 9, September 2002, pp. 722-726, ISSN 0008-5286 . PMID 12240535 . PMC 339565 (free full text).
- ↑ See Kosuch, pp. 25-27.
- ↑ a b cf. Lukoschus, p. 25 f.
- ^ A b c Center for Food Security & Public Health, Iowa State University: Glanders . (PDF; 121 kB). Retrieved March 2, 2013.
- ↑ Robert von Ostertag: The current status of the zoonosis question. In: Virchow's archive. Volume 275, No. 1, 1930, p. 596.
- ↑ See Lührs, p. 642.
- ↑ Cf. Gregory & Waag, p. 128.
- ↑ See Mießner, p. 14.
- ^ WC Nierman et al .: Structural flexibility in the Burkholderia mallei genome. In: Proceedings of the National Academy of Sciences . Volume 101, Number 39, September 2004, pp. 14246-14251, ISSN 0027-8424 . doi: 10.1073 / pnas.0403306101 . PMID 15377793 . PMC 521142 (free full text).
- ↑ See W. Drommer: Rotz (Malleus). In: Leo-Clemens Schulz (ed.): Pathology of domestic animals. Volume II: Diseases and Syndromes. Gustav Fischer, Jena, 1991, p. 19.
- ↑ See Ernst Joest (Ed.): Special pathological anatomy of domestic animals. Third volume. Richard Schoetz, Berlin 1924, pp. 788-791.
- ↑ See Dietz, p. 659.
- ↑ See Joest, p. 577.
- ↑ See Drommer, p. 19.
- ↑ See Lührs, p. 656.
- ↑ A. Goodyear, L. Kellihan, H. Bielefeldt-Ohmann, R. Troyer, K. Dean, S. Dow: Protection from pneumonic infection with Burkholderia species by inhalational immunotherapy. In: Infection and Immunity. Volume 77, Number 4, April 2009, pp. 1579-1588, ISSN 1098-5522 . doi: 10.1128 / IAI.01384-08 . PMID 19179415 . PMC 2663177 (free full text).
- ↑ See Lührs, p. 654.
- ↑ a b See Haubner, p. 420.
- ↑ See Mießner, p. 12.
- ↑ See Friedberger & Fröhner, p. 449 f.
- ↑ See Haubner, p. 419.
- ↑ See Haubner, p. 423.
- ↑ a b See Friedberger & Fröhner, p. 451.
- ↑ See Haubner, p. 49.
- ↑ See Center for Food Security & Public Health, Iowa State University: Glanders (PDF; 121 kB)
- ↑ Cf. Friedberger & Fröhner, p. 452.
- ↑ See Dr. Krüger-Hanssen: About snot contagium . Yearbooks of domestic and foreign entire medicine, Volume XIV, No. 2 (1837), p. 182 f.
- ↑ See Joest, p. 791 f.
- ↑ See Joest, p. 571.
- ↑ See Joest, p. 573.
- ↑ See Friedberger & Fröhner, p. 448.
- ^ See Friedberger & Fröhner, p. 454.
- ↑ Cf. Ferdinand Pieter Keyser: The diagnosis of snot on the carcass. Diss. (Vet. Med.) Bern 1910.
- ↑ See Mießner, p. 27.
- ↑ Mandy Elschner, Peggy Marten, Holger Scholz: Establishing an immunoblot procedure as a confirmation test for the complement fixation reaction in serological snot diagnostics. In: Summaries of the lectures and posters for the 26th AVID Conference - Bacteriology - Bad Staffelstein / Banz Monastery, 24. – 26. October 2007. Ed. of the German Veterinary Medical Society e. V. [o. O.] [o. J.]
- ↑ Cf. Christian Hallwachs: Treatise on the safe use of lime as a preventive agent against diseases of the lymphatic system, especially against snot and skin worms in the horse sex. [O. O.], 1822.
- ↑ a b Cf. Friedberger & Fröhner, p. 468.
- ↑ See Erich Kopkow: Treatment of a case of chronic snot in humans with snot vaccine. Diss. (Med.) Königsberg i. Pr. 1934.
- ↑ Snot. In: Meyers Konversations-Lexikon . 1888.
- ↑ Cf. C. Küttner: Contribution to the question about snot in humans. Virchow's Archives, Volume 39, No. 4 (1867), p. 583.
- ↑ Ghulam Muhammad, M. Zargham Khan, Muhammad Athar: Clinico-Micro Biological and Therapeutic Aspects of Glanders in Equines. In: Journal of Equine Science. 9, pp. 93-96, doi: 10.1294 / jes.9.93 .
- ↑ Cf. Gregory & Waag, p. 139 f.
- ^ Marianne Abele-Horn: Antimicrobial Therapy. Decision support for the treatment and prophylaxis of infectious diseases. With the collaboration of Werner Heinz, Hartwig Klinker, Johann Schurz and August Stich, 2nd, revised and expanded edition. Peter Wiehl, Marburg 2009, ISBN 978-3-927219-14-4 , p. 160.
- ↑ a b Hermann-Josef Petermann and Susanne Diekmann: Modernization of the snot investigation guidelines. In: Deutsches Tierärzteblatt. (Dt. TÄbl.) 60, 2012, pp. 908-914.
- ^ Wilhelm Rieck : On the pathology of horse diseases in the Middle Ages. In: Gundolf Keil , Rainer Rudolf, Wolfram Schmitt, Hans J. Vermeer (eds.): Specialist literature of the Middle Ages. Festschrift Gerhard Eis . Stuttgart 1968, pp. 277-292.
- ↑ See Friedberger & Fröhner, p. 437 f.
- ↑ See von den Driesch, p. 173.
- ↑ See Nadja Kosuch: Tierseuchen and their control on the Mittelweser in the mirror Nienburger sources (17th – 19th century) . Diss. (Med. Vet.) Hanover. Tenea Wissenschaft, Berlin 2004, p. 26 f.
- ↑ Cf. Petra Sedlmeier: From the extraordinary meat inspection to the control of self-control. Development of veterinary food monitoring in Baden and Württemberg. Diss. (Med. Vet.) [Gießen]. VVB Laufersweiler, Wettenberg 2005, p. 8.
- ^ Ordinance of Carl I, Duke of Braunschweig-Lüneburg (1713–1780, Reg.-Antr. 1735, nephew of Emperor Charles VI and brother-in-law of Frederick the Great, founder of the Collegium Carolinum ), subjecting horse snot to registration. Given in Braunschweig, April 4, 1771. Source: luederhniemeyer.com
- ↑ J. Raritsch: A few words about the pathogenesis of snot and worm disease in horses. Virchows Archiv, Volume 1–2 (1862), p. 33 ff.
- ↑ See Küttner, p. 559.
- ↑ a b cf. von den Driesch, p. 174.
- ↑ See Meyers Konversationslexikon 1888.
- ↑ Cf. The Medical Police in the Prussian States. A handbook for police and medical officials, namely doctors, surgeons, obstetricians, pharmacists and veterinarians. Edited by Walther and Zeller, Second Theil, published by Gottfried Basse, Quedlinburg / Leipzig 1830, p. 573 f. and p. 595 f.
- ↑ Cf. G. Jeanselme: Case of acute snot and worm in humans. Yearbook of all medicine at home and abroad. Volume 17, 1838, pp. 175-177.
- ↑ a b c Cf. Gregory & Waag, p. 123.
- ↑ See Derbyshire, pp. 722 ff.
- ↑ See Derbyshire, Conclusions .
- ^ See Friedberger & Fröhner, p. 442.
- ↑ See von Ostertag, p. 596 f.
- ↑ See Koch, p. 8.
- ↑ See Koch, p. 10.
- ↑ See Koch, p. 25 f.
- ↑ See Koch, p. 29.
- ↑ See Koch, p. 33.
- ^ RD Verma: Glanders in India with special reference to incidence and epidemiology. In: Indian Veterinary Journal , 1981, Volume 58, Number 3, pp. 177-183.
- ^ Bioterrorism Agents / Diseases. Centers for Disease Control and Prevention.
- ↑ a b Snot and Anthrax . In: Der Spiegel . No. 30 , 1998, pp. 55 ( online ).
- ↑ a b c Cf. Gregory & Waag, p. 122.