Matairesinol: Difference between revisions
No edit summary |
No edit summary |
||
| (14 intermediate revisions by 10 users not shown) | |||
| Line 3: | Line 3: | ||
| Watchedfields = changed |
| Watchedfields = changed |
||
| verifiedrevid = 462100478 |
| verifiedrevid = 462100478 |
||
| Reference = <ref>[http://www.sigmaaldrich.com/ |
| Reference = <ref>[http://www.sigmaaldrich.com/US/en/product/sial/40043 Matairesinol] at [[Sigma-Aldrich]]</ref> |
||
| Name = Matairesinol |
| Name = Matairesinol |
||
| ImageFile = Matairesinol.png |
| ImageFile = Matairesinol.png |
||
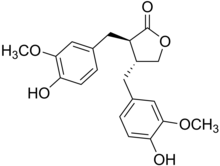
| ImageName = Chemical structure of matairesinol |
| ImageName = Chemical structure of matairesinol |
||
| IUPACName = ( |
| IUPACName = (8β,8′α)-4,4′-Dihydroxy-3,3′-dimethoxylignano-9,9′-lactone |
||
| SystematicName = (3''R'',4''R'')-3,4-Bis[(4-hydroxy-3-methoxyphenyl)methyl]oxolan-2-one |
|||
| OtherNames = (αR,βR)-α,β-Bis(4-hydroxy-3-methoxybenzyl)butyrolactone |
| OtherNames = (αR,βR)-α,β-Bis(4-hydroxy-3-methoxybenzyl)butyrolactone |
||
|Section1={{Chembox Identifiers |
|Section1={{Chembox Identifiers |
||
| Line 20: | Line 21: | ||
| InChIKey1 = MATGKVZWFZHCLI-LSDHHAIUSA-N |
| InChIKey1 = MATGKVZWFZHCLI-LSDHHAIUSA-N |
||
| CASNo = 580-72-3 |
| CASNo = 580-72-3 |
||
| CASNo_Ref = {{cascite| |
| CASNo_Ref = {{cascite|correct|CAS}} |
||
| CASNoOther = |
| CASNoOther = |
||
| UNII_Ref = {{fdacite|correct|FDA}} |
|||
| UNII = XLW63P8WUA |
|||
| PubChem = 119205 |
| PubChem = 119205 |
||
| ChEBI_Ref = {{ebicite|changed|EBI}} |
| ChEBI_Ref = {{ebicite|changed|EBI}} |
||
| Line 29: | Line 32: | ||
| DrugBank_Ref = {{drugbankcite|correct|drugbank}} |
| DrugBank_Ref = {{drugbankcite|correct|drugbank}} |
||
| DrugBank = DB04200 |
| DrugBank = DB04200 |
||
| KEGG_Ref = {{keggcite|correct|kegg}} |
|||
| KEGG = C10682 |
|||
| SMILES = O=C2OC[C@H](Cc1cc(OC)c(O)cc1)[C@H]2Cc3ccc(O)c(OC)c3 |
| SMILES = O=C2OC[C@H](Cc1cc(OC)c(O)cc1)[C@H]2Cc3ccc(O)c(OC)c3 |
||
| MeSHName = |
| MeSHName = |
||
| Line 42: | Line 47: | ||
}} |
}} |
||
'''Matairesinol''' is an [[organic compound]]. It is classified as a [[lignan]], i.e., a type of [[phenylpropanoid]]. It is present in some cereals, such as [[rye]], and together with [[secoisolariciresinol]] has attracted much attention for its beneficial nutritional effects.<ref>{{Ullmann|doi=10.1002/14356007.a06_093.pub2|title=Cereals|year=2006|last1=Seibel|first1=Wilfried|last2=Kim Chung|first2=Okkyung|last3=Weipert|first3=Dorian|last4=Park|first4=Seok-Ho|isbn=3527306730}}</ref> |
|||
'''Matairesinol''' is a plant [[lignan]]. It occurs with [[secoisolariciresinol]] in numerous foods such as oil seeds, whole grains, vegetables, and fruits.<ref>{{cite journal |vauthors=Niemeyer HB, Honig DM, Kulling SE, Metzler M |title=Studies on the metabolism of the plant lignans secoisolariciresinol and matairesinol |journal=J. Agric. Food Chem. |volume=51 |issue=21 |pages=6317–25 |date=October 2003 |pmid=14518962 |doi=10.1021/jf030263n }}</ref> |
|||
==Metabolism== |
|||
The plant lignans are precursors of the enterolignans (mammalian lignans). A number of plant lignans are metabolized to the enterolignans ([[enterodiol]] and [[enterolactone]]) that can potentially reduce the risk of certain cancers and cardiovascular diseases.<ref>{{cite journal |doi=10.1079/BJN20051371 |vauthors=Milder IE, Arts IC, van de Putte B, Venema DP, Hollman PC |title=Lignan contents of Dutch plant foods: a database including lariciresinol, pinoresinol, secoisolariciresinol and matairesinol |journal=Br. J. Nutr. |volume=93 |issue=3 |pages=393–402 |date=March 2005 |pmid=15877880 | |
The plant lignans are precursors of the enterolignans (mammalian lignans).<ref>{{cite journal |vauthors=Niemeyer HB, Honig DM, Kulling SE, Metzler M |title=Studies on the metabolism of the plant lignans secoisolariciresinol and matairesinol |journal=J. Agric. Food Chem. |volume=51 |issue=21 |pages=6317–25 |date=October 2003 |pmid=14518962 |doi=10.1021/jf030263n }}</ref> A number of plant lignans are metabolized to the enterolignans ([[enterodiol]] and [[enterolactone]]) that can potentially reduce the risk of certain cancers and cardiovascular diseases.<ref>{{cite journal |doi=10.1079/BJN20051371 |vauthors=Milder IE, Arts IC, van de Putte B, Venema DP, Hollman PC |title=Lignan contents of Dutch plant foods: a database including lariciresinol, pinoresinol, secoisolariciresinol and matairesinol |journal=Br. J. Nutr. |volume=93 |issue=3 |pages=393–402 |date=March 2005 |pmid=15877880 |doi-access=free }}</ref> |
||
==Biomedical considerations== |
|||
Although |
Although some studies attribute disease preventative (cardio-protective and hormone associated cancers like breast cancer) benefits of lignans, the results are inconclusive.<ref>[http://lpi.oregonstate.edu/infocenter/phytochemicals/lignans Linus Pauling Institute at Oregon State University<!-- Bot generated title -->]</ref> Matairesinol has been found to act as an [[agonist]] of the [[adiponectin receptor 1]] (AdipoR1).<ref name="pmid23691032">{{cite journal | vauthors = Sun Y, Zang Z, Zhong L, Wu M, Su Q, Gao X, Zan W, Lin D, Zhao Y, Zhang Z | title = Identification of adiponectin receptor agonist utilizing a fluorescence polarization based high throughput assay | journal = PLOS ONE | volume = 8 | issue = 5 | pages = e63354 | year = 2013 | pmid = 23691032 | pmc = 3653934 | doi = 10.1371/journal.pone.0063354 | bibcode = 2013PLoSO...863354S | doi-access = free }}</ref> |
||
== References == |
== References == |
||
| Line 52: | Line 59: | ||
{{Lignans}} |
{{Lignans}} |
||
{{Signaling peptide/protein receptor modulators}} |
|||
[[Category:Adiponectin receptor agonists]] |
[[Category:Adiponectin receptor agonists]] |
||
Latest revision as of 11:44, 18 January 2024
| |
| Names | |
|---|---|
| IUPAC name
(8β,8′α)-4,4′-Dihydroxy-3,3′-dimethoxylignano-9,9′-lactone
| |
| Systematic IUPAC name
(3R,4R)-3,4-Bis[(4-hydroxy-3-methoxyphenyl)methyl]oxolan-2-one | |
| Other names
(αR,βR)-α,β-Bis(4-hydroxy-3-methoxybenzyl)butyrolactone
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| DrugBank | |
| KEGG | |
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C20H22O6 | |
| Molar mass | 358.390 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Matairesinol is an organic compound. It is classified as a lignan, i.e., a type of phenylpropanoid. It is present in some cereals, such as rye, and together with secoisolariciresinol has attracted much attention for its beneficial nutritional effects.[2]
Metabolism[edit]
The plant lignans are precursors of the enterolignans (mammalian lignans).[3] A number of plant lignans are metabolized to the enterolignans (enterodiol and enterolactone) that can potentially reduce the risk of certain cancers and cardiovascular diseases.[4]
Biomedical considerations[edit]
Although some studies attribute disease preventative (cardio-protective and hormone associated cancers like breast cancer) benefits of lignans, the results are inconclusive.[5] Matairesinol has been found to act as an agonist of the adiponectin receptor 1 (AdipoR1).[6]
References[edit]
- ^ Matairesinol at Sigma-Aldrich
- ^ Seibel, Wilfried; Kim Chung, Okkyung; Weipert, Dorian; Park, Seok-Ho (2006). "Cereals". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a06_093.pub2. ISBN 978-3527306732.
- ^ Niemeyer HB, Honig DM, Kulling SE, Metzler M (October 2003). "Studies on the metabolism of the plant lignans secoisolariciresinol and matairesinol". J. Agric. Food Chem. 51 (21): 6317–25. doi:10.1021/jf030263n. PMID 14518962.
- ^ Milder IE, Arts IC, van de Putte B, Venema DP, Hollman PC (March 2005). "Lignan contents of Dutch plant foods: a database including lariciresinol, pinoresinol, secoisolariciresinol and matairesinol". Br. J. Nutr. 93 (3): 393–402. doi:10.1079/BJN20051371. PMID 15877880.
- ^ Linus Pauling Institute at Oregon State University
- ^ Sun Y, Zang Z, Zhong L, Wu M, Su Q, Gao X, Zan W, Lin D, Zhao Y, Zhang Z (2013). "Identification of adiponectin receptor agonist utilizing a fluorescence polarization based high throughput assay". PLOS ONE. 8 (5): e63354. Bibcode:2013PLoSO...863354S. doi:10.1371/journal.pone.0063354. PMC 3653934. PMID 23691032.
