1,2-dioxin
| Structural formula | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
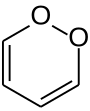
|
||||||||||
| General | ||||||||||
| Surname | 1,2-dioxin | |||||||||
| other names |
|
|||||||||
| Molecular formula | C 4 H 4 O 2 | |||||||||
| External identifiers / databases | ||||||||||
|
||||||||||
| properties | ||||||||||
| Molar mass | 84.07 g mol −1 | |||||||||
| safety instructions | ||||||||||
|
||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||
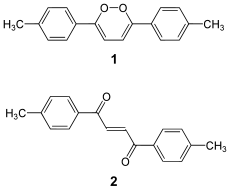
1,2-dioxin , also o dioxine , is a very unstable oxygen-containing heterocyclic organic compound which has not yet been isolated. Even substituted derivatives are very labile, for example 1,4-diphenyl-2,3-benzodioxin.
(no) stable 1,2-dioxin
In 1990, 3,6-bis ( p -tolyl) -1,2-dioxin was mistaken for the first stable derivative. However, it has been shown that the compound is not a derivative of a 1,2-dioxin (1) , but a (thermodynamically much more stable) dione (2) .
Stable derivatives
If the oxygen groups are replaced with sulfur groups, so-called 1,2-dithiines are obtained . These are often natural substances and have an antimicrobial effect , such as the thiarubrins . 1,2-Diselenine could also be isolated. Finally, the isomer 1,4-dioxin ( p -dioxin ), in contrast to 1,2-dioxin, is also stable.
Individual evidence
- ↑ This substance has either not yet been classified with regard to its hazardousness or a reliable and citable source has not yet been found.
- ^ JP Smith, AK Schrock, GB Schuster: Chemiluminescence of organic peroxides. Thermal generation of an o-xylylene peroxide , in: J. Am. Chem. Soc. 1981 , 104 , 1041-1047; doi : 10.1021 / ja00368a021 .
- ^ HJ Shine, DC Zhao: Electron transfer to excited doublet states. Photoirradiation of 10-methylphenothiazine cation radical perchlorate in solutions of phenylacetylene and p-tolylacetylene in acetonitrile , in: J. Org. Chem. 1990 , 55 , 4086-4089; doi : 10.1021 / jo00300a026 .
- ↑ E. Block, Z. Shan, RS Glass, J. Fabian: Revised structure of a purported 1,2-dioxin: a combined experimental and theoretical study , in: J. Org. Chem. 2003 , 68 , 4108-4111; PMID 12737603 ; doi : 10.1021 / jo034305i .