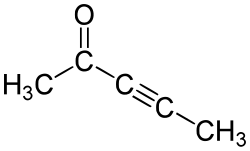
3-penty-2-one
| Structural formula | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|||||||||||||
| General | |||||||||||||
| Surname | 3-penty-2-one | ||||||||||||
| other names |
2-penty-4-one |
||||||||||||
| Molecular formula | C 5 H 6 O | ||||||||||||
| External identifiers / databases | |||||||||||||
|
|||||||||||||
| properties | |||||||||||||
| Molar mass | 82.10 g mol −1 | ||||||||||||
| safety instructions | |||||||||||||
|
|||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | |||||||||||||
3-Pentin-2-one (also: 2-Pentin-4-one ) is an organic compound with the empirical formula C 5 H 6 O. It belongs to the ketones and contains a C≡C triple bond .
The compound is formed as an intermediate in the stereo inversion of 3-pentyn-2-ol .
literature
- P. Carlier, G. Mouvier: Etude par spectrometrie de photoelectrons de la structure electronique des ynals et des ynones conjugues , J. Electron Spectrosc. Relat. Phenom. , 1979, 17, pp. 169-180; doi: 10.1016 / 0368-2048 (79) 85038-0 .
Web links
- Entry for 2-Pentyn-4-one . In: P. J. Linstrom, W. G. Mallard (Eds.): NIST Chemistry WebBook, NIST Standard Reference Database Number 69 . National Institute of Standards and Technology , Gaithersburg MD, accessed January 5, 2020.
Individual evidence
- ↑ This substance has either not yet been classified with regard to its hazardousness or a reliable and citable source has not yet been found.
- ↑ Jun Ogawa, Sheng-Xue Xie, Sakayu Shimizu: Production of (R) -3-pentyn-2-ol through stereoinversion of racemic 3-pentyn-2-ol by Nocardia fusca AKU 2123 , Applied Microbiology and Biotechnology , 1999, 52 , Pp. 327-331; doi: 10.1007 / s002530051527 .
- ↑ Jun Ogawa, Sheng-Xue Xie, Sakayu Shimizu: Stereoinversion of optically active 3-pentyn-2-ol by Nocardia species , Biotechnology Letters , 1999, 21, pp. 331-335; doi: 10.1023 / A: 1005484731976 .