Algar-Flynn-Oyamada reaction
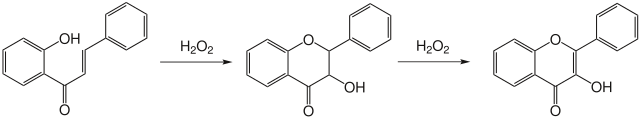
The Algar-Flynn-Oyamada reaction is a chemical reaction from the field of organic chemistry . It is used for the synthesis of flavonols ( derivatives of flavones with an additional hydroxyl group ) from chalcones . The reaction proceeds under oxidizing conditions in a basic medium with hydrogen peroxide as the oxidizing agent .
mechanism
Several mechanisms are possible to explain the reaction, but which the reaction follows has not yet been elucidated. It is known that there is a two-stage mechanism in which a dihydroflavonol is first formed, which is then oxidized to flavonol. Those mechanisms which run via an epoxidic intermediate product that can be formed by the oxidation of the double bond with hydrogen peroxide in a Weitz-Scheffer epoxidation can be excluded . There are therefore two possibilities as probable mechanisms:
- The nucleophilic attack of the phenolate formed by the base on the double bond with direct attack on the hydrogen peroxide.
- Nucleophilic attack of the phenolate with the formation of an enolate , which then attacks the hydrogen peroxide.
Individual evidence
- ↑ Joseph Algar, John P. Flynn: A new method for the synthesis of flavonols. In: Proceedings of the Royal Irish Academy. Section B: Biological, Geological, and Chemical Science. 42, 1934, pp. 1-8.
- ↑ T. Oyamada: A New General Method for the Synthesis of Flavonolderivatives. In: J. Chem. Soc. Japan . 55, No. 12, 1934, pp. 1256-1260.
- ↑ H. Wagner, I. Maurer, L. Farkas, J. Strelisky: Synthesis of polyhydroxy-flavonol methyl ethers with potential cytotoxic activity - I: Synthesis of quercetagetin and gossypetin dimethyl ethers for structural evidence of new flavonols from parthenium, chrysosplenium, larrea and spinacia species. In: Tetrahedron . 33, No. 11, 1977, pp. 1405-1409, doi : 10.1016 / 0040-4020 (77) 84092-1 .
- ^ TR Gormley, WI O'Sullivan: Flavanoid epoxides — XIII: Acid and base catalysed reactions of 2′-tosyloxychalcone epoxides. Mechanism of the algar-flynn-oyamada reaction. In: Tetrahedron. 29, No. 2, 1973, pp. 369-373, doi : 10.1016 / S0040-4020 (01) 93304-6 .