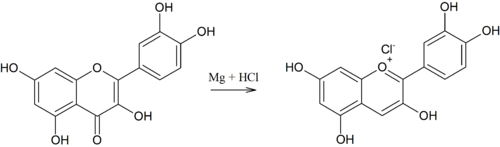Anthocyanidins
Anthocyanidins represent the coloring part of anthocyanins (a group of plant pigments ). This group consists of anthocyanidins, the aglycon , and glycosidically bound sugars. The spectrum of naturally formed sugars results in a variety of approx. 250 anthocyanins.
history
Richard Willstätter , who received the Nobel Prize for Chemistry in 1915 for his systematic pioneering work in this field , made a significant contribution to the elucidation of the structure of anthocyanins . He succeeded in isolating the anthocyanins from plant extracts and in releasing and identifying the anthocyanidins from them through degradation reactions. Ultimately, only about ten anthocyanidins are known to be natural parent compounds.
structure
The glycosidic bond in anthocyanins can be cleaved with acid catalysis and thus the anthocyanidin can be released. Anthocyanidins have multiple hydroxy-2-phenyl chromenyliumsalze (flavylium salts), as a counter ion of benzo pyrylium salts act in nature usually carboxylates of various water-soluble acids in laboratory preparations often chloride . Picrates used to be used for crystallization . Cyanidin is by far the most important in this plant pigment group , followed by delphinidin and pelargonidin . Their methyl ethers peonidin , petunidin and malvidin are also found frequently. Myrtillin is a glycoside of the delphinidine.
| Anthocyanidin | CAS (a) | Basic structure (R 3 = −OH) | R 1 , R 2 | - | R 5 | R 6 | R 7 | - | λ max (b) | - | pK S 1 (c) | pK S 2 | pK S 3 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Guibourtinidine (d) | 13544-54-2 |

|
−H, −H | −H | −H | −OH | 504 nm | .... | .... | .... | ||||
| Fisetinidine (d) | 2948-76-7 | −OH, −H | −H | −H | −OH | 520 nm | .... | .... | .... | |||||
| Robinetinidine (d) | 3020-09-5 | -OH, -OH | −H | −H | −OH | 532 nm | .... | .... | .... | |||||
| Pelargonidin (d) | 134-04-3 | −H, −H | −OH | −H | −OH | 523 nm | nb | .... | .... | |||||
| Cyanidine (d) | 528-58-5 | −OH, −H | −OH | −H | −OH | 538 nm | 2.98 ± 0.05 | 7.5 | .... | |||||
| Peonidin (paeonidin) | 134-01-0 | -OCH 3 , -H | −OH | −H | −OH | 536 nm | 2.09 ± 0.10 | 6.8 | .... | |||||
| Rosinidine | 4092-64-2 | -OCH 3 , -H | −OH | −H | −OCH 3 | .... | .... | .... | ||||||
| Delphinidin (d) | 528-53-0 | -OH, -OH | −OH | −H | −OH | 548 nm | 1.56 ± 0.20 | 5.85 | .... | |||||
| Pulchellidine | 19077-86-2 | -OH, -OH | −OCH 3 | −H | −OH | .... | .... | .... | ||||||
| Petunidin | 1429-30-7 | -OCH 3 , -OH | −OH | −H | −OH | 547 nm | .... | .... | .... | |||||
| Europinidine | 19077-87-3 | -OCH 3 , -OH | −OCH 3 | −H | −OH | .... | .... | .... | ||||||
| Malvidin | 643-84-5 | -OCH 3 , -OCH 3 | −OH | −H | −OH | 546 nm | 1.76 ± 0.07 | 5.36 ± 0.04 | 8.39 ± 0.07 | |||||
| Capensinidin | 19077-85-1 | -OCH 3 , -OCH 3 | −OCH 3 | −H | −OH | .... | .... | .... | ||||||
| Hirsutidine | 4092-66-4 | -OCH 3 , -OCH 3 | −OH | −H | −OCH 3 | .... | .... | .... | ||||||
| Aurantinidine (d) (e) | 25041-66-1 | −H, −H | −OH | −OH | −OH | .... | .... | .... | ||||||
| Quercetagetinidine (d) (f) | 42529-06-6 | −OH, −H | −OH | −OH | −OH | 519 nm | .... | .... | .... | |||||
| 6-hydroxydelphinidin (d) | 178436-68-5 | -OH, -OH | −OH | −OH | −OH | .... | .... | .... | ||||||
|
(a) CAS numbers of the chlorides.
(b)UV absorption in methanol with 0.1% HCl. Anthocyanidins absorb light about 10 nm longer than anthocyanidins glycosides ( bathochromic shift).
(c)pK S values of various anthocyanidin glycosides
(d) unsubstituted parent compounds
(e) 6-hydroxypelargonidine
(f) 6-hydroxycyanidine
|
||||||||||||||
Anthocyanidins always have a p-hydroxyphenyl substituent (B ring) in position 2 and a hydroxyl group in position 3 . The most important natural anthocyanidins are hydroxy-substituted in the 5- and 7-position of the A ring.
In addition to the group of anthocyanidins, there is also the small group of 3-deoxyanthocyanidins (with R 3 = H). Which includes
- Apigeninidin (3-deoxypelargonidin, Gesneridin)
- Luteolinidine (3-deoxycyanidine) and, as methyl ether, diosmetinidine (3-deoxypeonidine)
- Tricetinidine (3-deoxydelphinidine)
- Columnidin
3-Deoxyanthocyanidins behave like anthocyanidins in terms of color and acidity .
properties
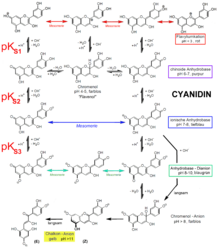
Anthocyanidins are sensitive to light, air and temperature; at pH values below 3 they are most stable in the form of their flavylium salts.
Anthocyanidins absorb light in the visible range between 450 and 650 nm and therefore appear red, purple or blue. The wavelength range is influenced not only by the molecular structure but also by the pH of the solution. In the acidic environment, the red color predominates, in the alkaline environment, blue and violet tones are to be found.
pH dependence of the color change
The color changes are based on chemical reactions .
- At pH values below 3 they are colored red and are in the form of flavylium cations .
- pH values between 4 and 5 lead to colorless carbinol pseudobases ("leuco bases", chromenol) due to hydroxylation .
- At pH values between 5 and 7, they are present as flavenols with a quinoid structure and are purple.
- At pH values between 7 and 8, this molecule is deprotonated to form the flavenolate anion , which is blue in color. Here the Π-electrons in the entire molecule are delocalized over the longest possible distance and can therefore be excited with the lowest possible light energy.
- If there are no glycoside groups in the 5-position, pH values of 8 and above also lead to flavenolate dianions, but in the alkaline environment the hydrolytic opening of the pyran ring competes . The molecule is irreversibly converted to a yellow chalcone anion.
- Compared with the pK S values of dihydroxybenzenes (pK S about 9.5 and 11.7) are anthocyanidins more than 1000 times more acidic. Flavylium salts in water are more than 10 times more acidic than formic acid (pK S 3.8).
synthesis
In 1921, based on the analysis of the substitution pattern of the flavonoids, Robert Robinson came to the conclusion that the flavonoids and thus the anthocyanidins must be biochemically composed of a C6 and a C6-C3 unit. In 1953 Arthur Birch and FW Donovan expanded this theory: The flavonoid biosynthesis must start from a p-hydroxycinnamic acid and three acetate units , a polyketo acid is formed as an intermediate .
The anthocyanidins are often not synthetically produced directly, but via similar flavonoids . Flavones are redox equivalent with anthocyanidines, flavanones with 3-deoxyanthocyanidines. Numerous methods are known for reducing flavones, flavanones and flavonols to the corresponding anthocyanidins or 3-deoxyanthocyanidines.
Anthocyanidins
The anthocyanidins are preparatively accessible by a Knoevenagel condensation of 3,5-dimethoxy-substituted salicylaldehydes with α- methoxyacetophenones . The primary product tautomerizes with ring closure to form α- flavanol , which splits off water when acid is added and results in the methoxy-substituted flavylium salt ( Robinson annulation ). Methyl ether groups can be split off gently with hydriodic acid .
Flavones
The Allan-Robinson condensation and its intramolecular variant, the Baker-Venkataraman rearrangement , lead to flavones through base-catalyzed condensation reactions .
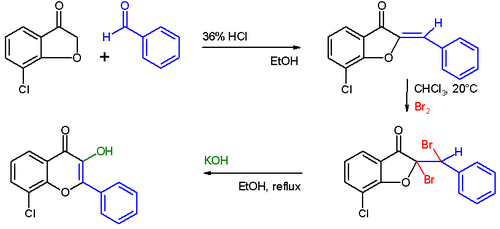
Flavonols
As early as 1908, by chance , Karl von Auwers discovered a ring expansion reaction ( Auwers reaction ), which leads from coumarones to flavonols .
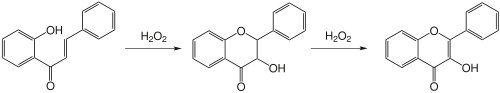
The Algar-Flynn-Oyamada reaction is a base-catalyzed ring closure reaction with hydrogen peroxide. Flavone intermediates are oxidized to flavonol .
reduction
With classic mild reduction methods, specific flavones can be reduced to 3-deoxyanthocyanidins or flavonols to anthocyanidins. With drastic reduction methods (sodium amalgam in water), however, flavanones can also be converted into red-colored flavylium salts.
Review article
- H. Halbwirth: "The Creation and Physiological Relevance of Divergent Hydroxylation Patterns in the Flavonoid Pathway", in Int J Mol Sci. 2010 , 11, 595-621 ( PMC 2852856 (free full text)).
Individual evidence
- ↑ R. Willstätter, AE Everest: “Investigations on the anthocyanins. I. About the coloring matter of the cornflower ”, Liebigs Ann. Chem. , 401 , 189-232 (1913) ( doi: 10.1002 / jlac.19134010205 ).
- ↑ R. Willstätter, TJ Nolan: “Studies on the anthocyanins. II. About the color of the rose ”, Liebigs Ann. Chem. , 408 , 1-14 (1915) ( doi: 10.1002 / jlac.19154080102 ).
- ↑ R. Willstätter, H. Mallison: "Investigations on the anthocyanins. III. About the color of the lingonberry ”, Liebigs Ann. Chem. , 408 , 15-41 (1915) ( doi: 10.1002 / jlac.19154080103 ).
- ^ R. Willstätter, EK Bolton: "Investigations on the anthocyanins. IV. About the color of the scarlet pelargonium ”, Liebigs Ann. Chem. , 408 , 42-61 (1915) ( doi: 10.1002 / jlac.19154080104 ).
- ↑ R. Willstätter, W. Mieg: “Investigations on the anthocyanins. V. About an anthocyanin of the delphinium ”, Liebigs Ann. Chem. , 408 , 61-82 (1915) ( doi: 10.1002 / jlac.19154080105 ).
- ↑ R. Willstätter, EH Zollinger: “Investigations on the anthocyanins. VI. About the colorants of grapes and blueberries ”, Liebigs Ann. Chem. , 408 , 83-109 (1915) ( doi: 10.1002 / jlac.19154080106 ).
- ↑ R. Willstätter, K. Martin: “Investigations on the anthocyanins. VII. About the dye of Althaea rosea ”, Liebigs Ann. Chem. , 408 , 110-121 (1915) ( doi: 10.1002 / jlac.19154080107 ).
- ↑ R. Willstätter, W. Mieg: “Investigations on the anthocyanins. VIII. About the dye of the wild mallow ”, Liebigs Ann. Chem. , 408 , 122-135 (1915) ( doi: 10.1002 / jlac.19154080108 ).
- ↑ R. Willstätter, TJ Nolan: “Studies on the anthocyanins. IX. About the dye of the peony ”, Liebigs Ann. Chem. , 408 , 136-146 (1915) ( doi: 10.1002 / jlac.19154080109 ).
- ↑ R. Willstätter, H. Mallison: "Investigations on the anthocyanins. X. About variations in flower colors, ” Liebigs Ann. Chem. , 408 , 147-162 (1915) ( doi: 10.1002 / jlac.19154080110 ).
- ^ R. Willstätter, EK Bolton: "Investigations on the anthocyanins. XI. About the anthocyanin of the red-flowering Salvia species ”, Liebigs Ann. Chem. , 412 , 113-136 (1917) ( doi: 10.1002 / jlac.19174120202 ).
- ↑ Entry on anthocyanidins . In: IUPAC Compendium of Chemical Terminology (the “Gold Book”) . doi : 10.1351 / goldbook.A00379 Version: 2.3.1.
- ↑ Analytical data for various anthocyanidins .
- ↑ Comparative representation of the VIS spectra .
- ↑ H. Pyysalo, O. Mäkitie: “Spectrometric Studies on the Acid Dissociation of Anthocyanins in Aqueous Solutions”, Acta Chem. Scand. , 1973 , 27 , 2681-2682 ( PDF ).
- ↑ Robert E. Asenstorfer, Patrick G. Iland, Max E. Tate, Graham P. Jones: "Charge equilibria and pK a of malvidine-3-glucoside by electrophoresis", Analytical Biochemistry , 2003 , 318 , 291-299 ( doi: 10.1016 / S0003-2697 (03) 00249-5 ).
- ^ Josh Hurwitz, Determining the Acid / Alkali and Color Properties of the Anthocyanin Delphinidin-3-Monoglucoside in Hydrangea Macrophylla . Ed .: University of Florida. ( weebly.com [PDF; 1000 kB ; accessed on February 20, 2016]).
- ↑ pK S 3 = 8.06 for apigeninidin determined by L. Costantino, G. Rastelli, M. Rossi and A. Albasini: "Quantitative measurement of proton dissociation and tautomeric constants of apigeninidin", J. Chem. Soc., Perkin Trans . 2 , 1995 , pp. 227-234 ( doi: 10.1039 / P29950000227 ).
- ↑ Natural substances as pH indicators .
- ↑ Yellow dyes absorb below 400 nm, cf. Quercetin , Chalcon at 350 nm.
- ↑ Chemical Structure of Anthocyanines - pH Dependency, p. 17 ( limited preview in the Google book search).
- ↑ Y. Asahina, G. Nakagome, M. Inubuse: “About the reduction of the flavone and flavanone derivatives (V. Communication on the flavanone glucosides)”, Ber. German Chem. Ges. , 1929 , 62 , pp. 3016-3021 ( doi: 10.1002 / cber.19290621112 ) and cited therein. Lit.
- ↑ TS Wheeler: Flavones In: Organic Syntheses . 32, 1952, p. 72, doi : 10.15227 / orgsyn.032.0072 ; Coll. Vol. 4, 1963, p. 478 ( PDF ).
- ↑ K. Auwers, K. Müller: "Conversion of Benzal-Coumaranonen in Flavonole", Ber. German Chem. Ges. , 41 , 4233-4241 (1908) ( doi: 10.1002 / cber.190804103137 ).
- ↑ K. v. Auwers, P. Pohl: “About the conversion of benzal coumaranones into flavonols”, Liebigs Ann. Chem. , 405 , 243-294 (1914) ( doi: 10.1002 / jlac.19144050302 ).
- ↑ K. v. Auwers, P. Pohl: “A synthesis of fisetins”, Ber. German Chem. Ges. , 48 , 85-90 (1915) ( doi: 10.1002 / cber.19150480114 ).
- ↑ K. v. Auwers: "On the formation of flavonols from benzal-coumaranones", Ber. German Chem. Ges. , 49 , 809-819 (1916) ( doi: 10.1002 / cber.19160490188 ).
- ↑ K. v. Auwers, E. Auffenberg: “About coumaranones and hydrindones”, Ber. German Chem. Ges. , 52 , 92-113 (1919) ( doi: 10.1002 / cber.19190520114 ).
- ↑ F. Tiemann , W. Will: "About the hesperidin, a glucoside of the Aurantiaceae, and his cleavage products", Ber. German Chem. Ges. , 14 , 946-974 (1881).