Malvidin
| Structural formula | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
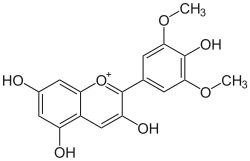
|
|||||||||||||
| Structure without anion (mostly chloride) | |||||||||||||
| General | |||||||||||||
| Surname | Malvidin | ||||||||||||
| other names |
3,4 ', 5,7-tetrahydroxy-3', 5'-dimethoxyflavylium |
||||||||||||
| Molecular formula | C 17 H 15 ClO 7 (chloride) | ||||||||||||
| Brief description |
red crystals with transmitted light and green with reflected light |
||||||||||||
| External identifiers / databases | |||||||||||||
|
|||||||||||||
| properties | |||||||||||||
| Molar mass | |||||||||||||
| Physical state |
firmly |
||||||||||||
| Melting point |
> 300 ° C (anhydrous) |
||||||||||||
| solubility |
soluble in ethanol |
||||||||||||
| safety instructions | |||||||||||||
|
|||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | |||||||||||||
Malvidin is a primary plant pigment that belongs to the group of flavonoids or the flavonoid subgroup of anthocyanidins . As an aglycon of many glycosides such as malvin (malvidin-3,5-diglucoside, an anthocyanin ), it is often found in nature and is responsible, among other things, for the color of red wine and the blue color in primroses .
properties
Malvidin is typically sold as chloride , a moderately water-soluble, reddish-brown powder. It irritates mucous membranes and eyes on contact .
Occurrence
Anthocyanins , the glycosides of the anthocyanidins, are plant pigments from the group of flavonoids. The water-soluble anthocyanins occur in almost all higher plants with red, purple, blue or even yellow color. At pH values below 4 they show a red color, between 4 and 5 they are colorless, at pH 6 to 7 they are purple, at 7 to 8 they are deep blue, and above 8 they are yellow.
Glycosides
A number of glycosides are known from malvidin :
- Malvin is the Malvidindi glucoside
- Oenin is the malvidin-3-glucoside
- Primulin is the malvidin-3- O - galactoside
- Malvidin-3-rutinosid is a pigment found in Curcuma alismatifolia ; Acylated malvidin-3-rutinoside are responsible for the purple color of Petunia integrifolia subsp. inflata
- Malvidin-3-O-glucoside-5-O- (6-acetylglucosid) is the pigment for the blue color of different Gerianien is responsible
Web links
Individual evidence
- ↑ a b c Entry on malvidin chloride. In: Römpp Online . Georg Thieme Verlag, accessed on February 7, 2019.
- ↑ Data sheet Malvidin chloride from Sigma-Aldrich , accessed on April 9, 2011 ( PDF ).
- ↑ M Nakayama, MS Roh, K Uchida, Y Yamaguchi, K Takano, M Koshioka: Malvidin 3-rutinoside as the pigment responsible for bract color in Curcuma alismatifolia . In: Bioscience, Biotechnology, and Biochemistry . 64, No. 5, 2000, pp. 1093-5. doi : 10.1271 / bbb.64.1093 . PMID 10879491 .
- ↑ F. Tatsuzawa: Acylated malvidin 3-rutinosides in dusky violet flowers of Petunia integrifolia subsp. Inflata . In: Phytochemistry . 52, No. 2, 1999, pp. 351-355. doi : 10.1016 / S0031-9422 (99) 00095-3 .
- ↑ Kenneth R. Markham, Kevin A. Mitchell, Murray R. Boase: Malvidin-3-O-glucoside-5-O- (6-acetylglucoside) and its color manifestation in 'Johnson's Blue' and other 'Blue' geraniums . In: Phytochemistry . 45, No. 2, 1997, pp. 417-423. doi : 10.1016 / S0031-9422 (96) 00831-X .