Primulin
| Structural formula | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
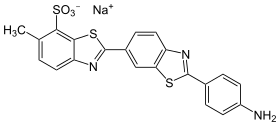
|
||||||||||||||||
| General | ||||||||||||||||
| Surname | Primulin | |||||||||||||||
| other names |
|
|||||||||||||||
| Molecular formula | C 21 H 14 N 3 NaO 3 S 3 | |||||||||||||||
| External identifiers / databases | ||||||||||||||||
|
||||||||||||||||
| properties | ||||||||||||||||
| Molar mass | 475.54 g mol −1 | |||||||||||||||
| Melting point |
> 300 ° C |
|||||||||||||||
| safety instructions | ||||||||||||||||
|
||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||
Primulin is a direct dye from the group of thiazole dyes , which contains two benzothiazole ring systems.
Extraction and presentation
Primulin yellow can be obtained by sulphonation of the primulin base . This can be represented by dehydrothiotoluidine from para-toluidine and sulfur.
use
Primulin has been known for dyeing cotton since the mid-19th century. It was first used in 1935 as a vital stain . Primulin is particularly good at staining pollen .
Individual evidence
- ↑ a b Datasheet Primuline, Dye content 50% from Sigma-Aldrich , accessed on November 7, 2014 ( PDF ).
- ↑ Rajbir Singh: Synthetic Dyes . Mittal Publications, 2002, ISBN 81-7099-832-8 , pp. 174 f . ( limited preview in Google Book search).
- ^ Rudolf Nietzki : Chemistry of the organic dyes . 1906, p. 289 ( limited preview in Google Book search).
- ↑ Paul Ruggli , Sal. Max Pestalozzi: About the cotton affinity of derivatives of dehydro-thiotoluidine and primulin. (V. Communication on dyeing processes) . In: Helvetica Chimica Acta . tape 9 , no. 1 , 1926, pp. 364–378 , doi : 10.1002 / hlca.19260090142 ( PDF ).
- ^ FWD Rost: Fluorescence Microscopy . Cambridge University Press, 1995, ISBN 0-521-41088-6 , pp. 350 f . ( limited preview in Google Book search).