Delphinidin
| Structural formula | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|

|
|||||||||||||||||||
| Figure without anion (mostly chloride) | |||||||||||||||||||
| General | |||||||||||||||||||
| Surname | Delphinidin | ||||||||||||||||||
| other names |
3,3 ', 4', 5,5 ', 7-hexahydroxy-flavylium |
||||||||||||||||||
| Molecular formula | C 15 H 11 O 7 (cation) | ||||||||||||||||||
| Brief description |
deep red prisms or needles with metallic sheen (chloride) |
||||||||||||||||||
| External identifiers / databases | |||||||||||||||||||
|
|||||||||||||||||||
| properties | |||||||||||||||||||
| Molar mass |
|
||||||||||||||||||
| Physical state |
firmly |
||||||||||||||||||
| Melting point |
> 350 ° C (chloride) |
||||||||||||||||||
| solubility |
Easily soluble in ethanol and ethyl acetate (chloride) |
||||||||||||||||||
| safety instructions | |||||||||||||||||||
|
|||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | |||||||||||||||||||
Delphinidin is an anthocyanin dye from red to purple in color.
Occurrence
Delphinidin is found in the flowers of the barnacle spur ( Delphinium consolida ) and the hydrangea ( Hydrangea ). It also occurs in the first blue rose cultivation, Blue Boy. In red fruits, cyanidin usually dominates , but delphinidin in black currants.
It occurs as dimethyl ether in malvidin (red wine).
properties
Delphinidin is a purple flower pigment of the anthocyanin group. The dye is not toxic. It dissolves in acids with a red color, with weak bases delphinidin separates with a purple hue.
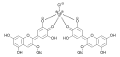
Hydrangeas form a blue color from delphinidin in the presence of aluminum or iron salts chelate complexes . To do this, these metal salts must be added to the garden soil using appropriate fertilizers.
Individual evidence
- ↑ a b c Entry on anthocyanins. In: Römpp Online . Georg Thieme Verlag, accessed on December 28, 2014.
- ↑ a b Data sheet Delphinidin chloride at Sigma-Aldrich , accessed on February 7, 2019 ( PDF ).