Pharmaceutical technology
The Pharmaceutical Technology is a scientific and technical department that deals with the production of drugs is concerned. An essential sub-area of pharmaceutical technology is the theory of dosage forms - dosage forms are preparations of medicinal substances in a certain form, for example as tablets .
An older term for the preparation and manufacture of medicines is galenics (named after the Greek doctor Galenos ). The term galenic is used in the traditional, artisanal production of pharmaceuticals as well as in modern pharmaceutical companies . The term pharmaceutical technology usually refers to modern drug production.
Dosage form
The preparation in which an active ingredient is administered is referred to as the drug form or dosage form. In the simplest case of the individually divided powder without auxiliaries, the active ingredient itself already represents the complete drug form. However, individually dosed powders, be it as a pure active ingredient or as a mixture of active ingredients and auxiliaries, hardly have a separate drug form due to the many disadvantages today Meaning. A dosage form therefore consists of active ingredients and auxiliary substances that are processed in a special way.
In addition to the actual active ingredient or mixture of active ingredients, the dosage form is of decisive importance for the effectiveness of the drug. It determines the essential properties of the finished pharmaceutical preparation (manufacture, storage, shelf life, pharmacokinetics , microbial purity, packaging, etc.). In order to correctly assess the effect of a drug, the dosage form must always be taken into account in addition to the pure active ingredient.
Requirements for a dosage form
There are many different requirements for a dosage form:
- Dosing accuracy
- chemical stability
- biological stability
- physical stability
- uniformity
- physiological compatibility
- Active ingredient content and active ingredient distribution
- Outer shape
- Appearance
- Concealing a bad smell, taste
Development of a dosage form
Initially, drug forms developed empirically, that is, through simple trial and error. What was useful was kept. This is how the “historical” dosage forms like the pill came about, which - apart from the name - no longer exist today. (Incidentally: What is now commonly referred to as the " pill " - that is, the oral contraceptives - are in most cases coated tablets or film-coated tablets.)
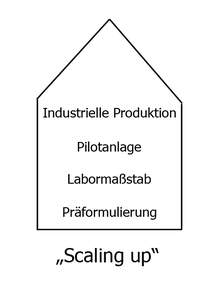
Today dosage forms are specifically developed based on scientific knowledge. At the beginning there is the “pre-formulation” comparable to a prototype. The next stage is to reproduce the dosage form on a small scale: "laboratory scale". This is followed by the first test facility and, at the end of the process, large-scale production. This process is known as “scaling-up” . Specific problems arise at each “enlargement level”: producing a tablet in the laboratory or in large quantities on rotary presses is a difference.
Preformulation Studies
The solubility of the active ingredient in an aqueous medium is of great importance. If the solubility is less than 0.5%, the absorption can be very poor, but sometimes a very high solubility is also poor. The pH value changes significantly when the active molecule moves from the gastrointestinal tract . By determining the pK a of a substance, the solubility of the substance in water can be calculated. Active ingredients can appear in different forms ( polymorphism ), which differ in terms of their solubility and stability.
Affecting the solubility
If, due to pH dependencies and polymorphism studies, good active ingredients are presumably poorly absorbable, the galenic specialist can apply physical-chemical and chemical measures to improve the solubility of an active ingredient. In physico-chemical processes, the active ingredient is often packaged in a hydrate-free environment (for example in a polyethylene glycol matrix). When the solid solution, the matrix, dissolves, the active ingredient is dispersed and can then be solvated. Another method is to warm the solvent so that more substance can be dissolved.
By introducing substituents that can be split off by enzymatic action, active ingredients are chemically changed. This serves to improve the solubility properties, to better overcome the blood-brain barrier , to prolong the effect of a substance, to reduce the bad taste. The active ingredient with a substituent is called a pro-drug. One example of a prodrug is phenacetin, which is converted into paracetamol by splitting off acetate. For example, succinic acid was introduced in methylprednisolone to improve its water solubility.
If acids and bases are present as active ingredients, salts can sometimes also be used.
Auxiliary materials
The pharmaceutical excipients used must not react with the active substance. The auxiliary materials must not pose a toxicological hazard. The factorial design is used to determine the stability of an active ingredient together with auxiliary materials . Here one examines an active ingredient with various auxiliary substances depending on the time. The active ingredients are stored for a long time at different temperatures and degrees of humidity. The decrease in the concentration of the active ingredient provides information on the stability of the active ingredient in an excipient.
Good Manufacturing Practice (GMP)
The literal translation of Good Manufacturing Practice is “ Good Manufacturing Practice ” (also “Good manners in producing”), which is usually expressed by the manufacturing companies and those affected as “ Give more paper ”.
The reason for the introduction of a general guideline on manufacturing practice was the desire to establish internationally recognized methods of proper pharmaceutical production in order to guarantee the harmlessness and safety of drugs, i.e. the pharmaceutical quality, over the entire life cycle of a drug.
The term begins with the extraction and the first processing steps of the raw materials (active ingredients and auxiliary materials) and ends with the expiry of the expiry date on the finished medicinal product. In general, this time span is around 3–5 years and depends on the type of substances used, the dosage form and the duration of the intermediate storage as well as the duration of the finished drug.
In 1986 the WHO introduced an initially non-binding guideline, which was revised and modernized in 1992. In the 1992 version, the “in-process control”, i.e. the precise adherence to and checking of the individual manufacturing steps, was given a lot of consideration. The reason for this is the insight that quality cannot be achieved through subsequent testing and checking, but only through proper manufacturing and quality control - in contrast to earlier - only finally determines the quality produced.
In 1970 the Pharmaceutical Inspection Convention (PIC), a guideline for the proper testing of drugs, was passed.
Since then, the EU has also passed its own directive (EG-GMP), which roughly corresponds to the PIC regulations. It consists of a binding guideline and a recommended part (guide).
Also worth mentioning are the SOP ( Standard Operating Procedures ), in which the individual manufacturing steps are precisely documented. They guarantee continuous transparency of the entire production process.
In other areas of pharmacy, these regulations are supplemented by corresponding guidelines:
- GLP - Good Laboratory Practice
- GCP - Good Clinical Practice
- GAMP - Good Automated Manufacturing Practice
The GMP and related guidelines cover the following topics, among others:
- Due diligence
- Training of staff
- Premises
- Separation of manufacturing, packaging and storage
- exam
- Labelling
- Hygiene, especially microbial contamination
- Quality of materials
- Rules for self-inspection and audits (external control)
- In-process control
- Validation
- Quality control
See also
literature
- Alfred Fahr : Pharmaceutical Technology. For studies and work . Deutscher Apotheker Verlag, Stuttgart 2015, 12th revised edition, ISBN 978-3-7692-6194-3 . Originally developed by Rudolf Voigt .
- David Schoebel: Multi-criteria design of pharmaceutical active ingredient plants. Gabler, Betriebswirtschaftlicher Verlag (2008), ISBN 3834912166