Atmospheric distillation
Atmospheric distillation ( lat. Distillate “dripping down” ) is a thermal separation process to obtain vaporizable liquids or to separate solvents from substances that are difficult to vaporize. The distillation is carried out at approximately atmospheric pressure (= mean air pressure), which at sea level is 101 325 Pa = 101.325 kPa = 1 013.25 hPa ( hectopascal = millibar ) according to the standard .
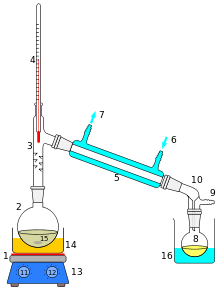
During the distillation, the starting mixture is first brought to the boil by supplying heat . The resulting vapor, which is made up of the various volatile components of the solution to be separated, is liquefied again by cooling in a condenser . The Liebig cooler is often used on a laboratory scale . The liquid condensate is then collected. Typical applications of distillation are the burning of alcohol and the distillation ( rectification ) of petroleum in the refinery or the production of distilled water .
Atmospheric distillation is the most commonly used case of isobaric distillation (= distillation at constant pressure by supplying heat) in contrast to isothermal distillation, which is less commonly used, by reducing pressure.
advantages
Atmospheric distillation has the advantage over vacuum distillation that there is no need to generate a vacuum inside the distillation system or distillation apparatus.
disadvantage
In atmospheric distillation, the thermal load on the starting mixture and the volatile components is higher than in vacuum distillation. The process is not well suited for the separation of high-boiling and temperature-sensitive mixtures of substances.
Individual evidence
- ^ Brockhaus ABC Chemie , VEB FA Brockhaus Verlag Leipzig 1965, pp. 277-279.