Blanc's rule
The Blanc rule is a rule of organic chemistry which was established in 1907 by HG Blanc. This rule states that aliphatic dicarboxylic acids with two and three methylene groups (i.e., five or fewer carbon atoms) form cyclic anhydrides when heated with dehydrating agents . In contrast, aliphatic dicarboxylic acids with four or more methylene groups (i.e. with six or more carbon atoms) form cycloalkanones according to Blanc's rule .
Examples
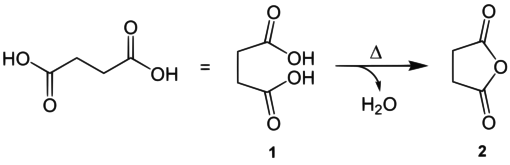
An example of a dicarboxylic acid with two methylene groups would be succinic acid ( 1 ). Succinic anhydride ( 2 ) is formed from 1 with elimination of water :
Other dicarboxylic acids that are subject to dehydration with cyclization are glutaric acid , maleic acid and phthalic acid . An example of a dicarboxylic acid with four methylene groups would be adipic acid ( 3 ). From 3 , with elimination of water and carbon dioxide, cyclopentanone ( 4 ) is formed:
Other dicarboxylic acids which form cyclic alkanones with elimination of water are pimelic acid and suberic acid .
Individual evidence
- ↑ Ed .: Jürgen Falbe ... arr. By Eckard Amelingmeier ... by Hermann Römpp]: Römpp-Lexikon Chemie / 1, A-Cl . 10., completely revised. Edition. Thieme, Stuttgart 1996, ISBN 3-13-734610-X , p. 462 .
- ↑ Blanc's rule. Retrieved June 4, 2019 .
- ^ A b c Siegfried Hauptmann: Organic chemistry: with 65 tables . 1st edition. Thun, Frankfurt am Main 1985, ISBN 3-87144-902-4 , p. 434 .