Bradsher cyclization
The Bradsher cyclization or Bradsher reaction is a name reaction in organic chemistry . It is named after its discoverer Charles K. Bradsher (* 1912), who published it in 1939. It is used for the synthesis of various polycyclic , aromatic compounds by acid-catalyzed cyclization.
The reaction takes place if one of the following functional groups is bound to the aromatic : the carbonyl group from ketones or aldehydes , nitriles , aldehyde derivatives, amino alcohols, halo ethers, olefins ( alkenes ) and many more.
mechanism
The mechanism of the Bradsher cyclization is exemplified using the reaction of 2 - ([1,1'-diphenyl] -2-yl) acetaldehyde:
The Bradsher cyclization with a carbonyl group proceeds in four steps. First, the carbonyl group 1 is protonated by adding an acid . The oxonium ion 2 is formed , which is attacked nucleophilically by the aromatic in the next step. Here is formed under closure of a six-membered ring an alcohol 3 . This is followed by the splitting off of a proton and thus a rearomatization of the former aromatic , resulting in alcohol 4 . In the last step, water is split off and phenanthrene 5 is formed as a product.
Normally, the Bradsher cyclization, as described here, proceeds with acid catalysis. However, there are also reports of reaction processes that were triggered with strong bases or with the help of radiation.
Applications
One of the most important uses of this reaction is in the manufacture of phenanthrenes and anthracene . Some examples of the Bradsher cyclization are shown below. These should give an impression of how far-reaching the possible applications of this reaction are.
Manufacture of anthracene
The following reaction shows how one can prepare anthracene or its derivatives with the help of the Bradsher cyclization . The starting material here is 2-benzylbenzaldehyde:
If the acyl group of a ketone is present in the educt instead of the formyl group (of the aldehyde ), an alkyl- or aryl-substituted anthracene is obtained as the product.
Unusual starting materials
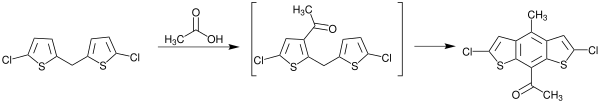
The following reaction shows the preparation of 1- (2,6-dichloro-4-methylbenzo [1,2- b : 5,4- b ' ] dithiophen-8-yl) ethan-1-one with the aid of the Bradsher cyclization . The starting material used is bis (5-chlorothiophen-2-yl) methane, which is acetylated with acetic acid under the influence of polyphosphoric acid . This reaction makes it clear that no Bradsher cyclization can be carried out on educts without one of the functional groups mentioned. First it is necessary to bring one of these groups to the molecule. Here, for example, an acetyl group is brought to the starting material by working up with acetic acid and the ketone formed as an intermediate is immediately cyclized with elimination of water .
Individual evidence
- ↑ a b c Jie Jack Li: Name Reactions. 4th edition, Springer, Heidelberg 2009, ISBN 978-3-642-01053-8 , pp. 66-67.
- ^ Charles K. Bradsher: Synthesis of Phenanthrene Derivatives. IV.1 9,10-Cyclopenteno- and 9,10-Cyclohexenophenanthrenes . In: Journal of the American Chemical Society . tape 61 , no. 11 , 1939, pp. 3131-3132 , doi : 10.1021 / ja01266a044 .
- ^ Charles K. Bradsher: Aromatic Cyclodehydration . 1 VII.2 Phenanthrene . In: journal of american chemical society . tape 62 , no. 10 , 1940, p. 2806-2807 , doi : 10.1021 / ja01867a051 .
- ^ Charles K. Bradsher, Winston J. Jackson Jr .: Aromatic Cyclodehydration. XXVIII.1 9,10-Dialkylphenanthrenes by Cyclization of Ketones . In: journal of american chemical society . tape 76 , no. 16 , 1954, pp. 4140-4143 , doi : 10.1021 / ja01645a026 .
- ^ A b Z. Wang: Comprehensive Organic Name Reactions and Reagents, 3 Volume Set . John Wiley & Sons, Hoboken, New Jersey 2009, ISBN 978-0-471-70450-8 , p. 501.
- ^ Z. Wang: Comprehensive Organic Name Reactions and Reagents, 3 Volume Set . John Wiley & Sons, Hoboken, New Jersey 2009, ISBN 978-0-471-70450-8 , p. 502.
- ^ CK Bradsher, Sidney T. Webster: A New Base-catalyzed Cyclization Reaction . In: journal of american chemical society . tape 79 , no. 2 , 1957, p. 393-395 , doi : 10.1021 / ja01559a042 .
- ^ Charles K. Bradsher, Lennard J. Wissow: Aromatic Cyclodehydration. XV.1 9,10-bis (p-hydroxyphenyl) phenanthrenes . In: journal of american chemical society . tape 65 , no. 12 , 1943, pp. 2304–2305 , doi : 10.1021 / ja01252a013 .
- ↑ M. Ahmed, John Ashby, O. Meth-Cohn: The direct Bradsher reaction: synthesis of benzodithiophens and related systems . In: J. Chem. Soc. D . tape 17 , 1970, pp. 1094-1095 , doi : 10.1039 / C29700001094 .

![Mechanism of the Bradsher cyclization using the example of 2 - ([1,1'-diphenyl] -2-yl) acetaldehyde](https://upload.wikimedia.org/wikipedia/commons/thumb/b/b5/Bradsher_Cyclosation_Mecha_Version_4.svg/620px-Bradsher_Cyclosation_Mecha_Version_4.svg.png)