Butanediol dehydrogenase
| Butanediol dehydrogenase | ||
|---|---|---|

|
||
| Ribbon model of the meso- 2,3-butanediol dehydrogenase from Klebsiella pneumoniae , according to PDB 1GEG | ||
| other names |
|
|
| Cofactor | NAD + | |
| Enzyme Classifications | ||
| EC, category | 1.1.1.4 , oxidoreductase | |
| Response type | Dehydration | |
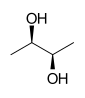
| Substrate | ( R , R ) -2,3-butanediol + NAD + | |
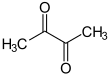
| Products | ( R ) -acetoin + NADH / H + | |
| EC, category | 1.1.1.76 , oxidoreductase | |
| Response type | Dehydration | |
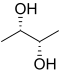
| Substrate | ( S , S ) -2,3-butanediol + NAD + | |
| Products | ( S ) -acetoin + NADH / H + | |
| Occurrence | ||
| Parent taxon | bacteria | |
Butanediol dehydrogenases are enzymes that catalyze the dehydrogenation of 2,3-butanediol to acetoin . These enzymes belong to the family of oxidoreductases , with the hydroxyl group acting as a donor and NAD + as an acceptor . In addition, the ( R , R ) -butanediol dehydrogenase takes part in the metabolism of butyric acid . The enantiomeric substrate with left-handed rotation is converted by ( S , S ) -butanediol dehydrogenase.
Stereoisomeric specificities of 2,3-butanediol dehydrogenases
2,3-Butanediol has a stereoisomeric specificity. Some dehydrogenases oxidize a hydroxy group in the D configuration, while other dehydrogenases oxidize a hydroxy group in the L configuration. Since the meso -2,3-butanediol contains hydroxyl groups in the D - (-) - and L - (+) - configuration, it serves as a substrate for all ( R , R ) -butanediol dehydrogenases. The configuration of acetoin, formed by the oxidation of meso -2,3-butanediol, depends on whether the hydroxyl group in the D - (-) or the L - (+) configuration is oxidized.
The bacterium Bacillus polymyxa contains D - (-) - 2,3-butanediol dehydrogenase. The 2,3-butanediol dehydrogenases of the bacteria Enterobacter aerogenes and Aeromonas hydrophila are L - (+) - dehydrogenases. Bacillus subtilis contains both D - (-) - and L - (+) - dehydrogenases. The configuration of the carbon atom of the 2,3-butanediol that is not oxidized affects the rate of oxidation. Bacteria that use meso- 2,3-butanediol, sodium acetate, or sodium lactate for energy metabolism contain D - (-) - dehydrogenases. In Enterobacter aerogenes , D - (-) - dehydrogenase is preferred as substrate with racemic acetoin, whereas L - (+) - dehydrogenase is preferred as substrate when using carbohydrates .
The presence of various combinations of D - (-) - and L - (+) - dehydrogenases and acetoin racemases can explain the appearance of three isomeric 2,3-butanediols as the end product of the fermentation of carbohydrates.
Catalyzed equilibrium
( R , R ) -2,3-butanediol is oxidized and dehydrated by the ( R , R ) -butanediol dehydrogenase. In addition to the reduction equivalent NADH , ( R ) -acetoin is formed.
( S , S ) -2,3-butanediol is oxidized and dehydrated by the ( S , S ) -butanediol dehydrogenase. In addition to the reduction equivalent NADH , ( S ) -acetoin is formed.
Irreversible reduction
( S , S ) -Butanediol dehydrogenase can also catalyze the irreversible reduction of diacetyl to ( S ) -acetoin:
Individual evidence
- ^ Mary B. Taylor, Elliot June: Stereoisomeric specificities of 2,3-butanediol dehydrogenases . In: Biochimica et Biophysica Acta . 39, No. 3, April 22, 1960, pp. 338-457. doi : 10.1016 / 0006-3002 (60) 90197-9 . PMID 13837186 .