Chiral derivatization reagent
Chiral derivatization reagents are used in analytical chemistry to characterize stereoisomers or stereoisomeric mixtures (mostly enantiomeric mixtures). A mixture of enantiomers is reacted with the enantiomerically pure chiral derivatizing reagent. This reaction gives diastereomers , the ratio of which can easily be determined analytically (NMR, HPLC, etc.). The enantiomer ratio in the sample to be examined can then be inferred from the diastereomer ratio. In NMR spectroscopy , the chiral derivatization reagents are also called chiral shift reagents.
history
Since the introduction of NMR spectroscopy in analytical chemistry, several methods of using chiral derivatizing reagents have been developed. The difference in the NMR spectra of diastereomers is measured quantitatively using the integrals of selected resonances . A mixture of enantiomers (enantiomers have the same NMR spectra) is thus converted into diastereomers which have different NMR spectra by means of a suitable enantiomerically pure chiral derivatization reagent. Harry S. Mosher et al. used the carboxylic acid chloride of ( R ) - or ( S ) -α-methoxy-α- (trifluoromethyl) phenylacetic acid, also known as Mosher acid , as a chiral derivatizing reagent for the investigation of the enantiomeric purity of alcohols and amines .
Since then, other similar processes have been developed, e.g. B. using phosphorus- and boron-containing enantiomerically pure chiral derivatizing reagents.
α-methoxy-α- (trifluoromethyl) phenylacetic acid
The carboxylic acid chloride of the enantiomerically pure ( R ) - or ( S ) -α-methoxy-α- (trifluoromethyl) phenylacetic acid has also been used to determine the absolute configuration of simple chiral amines or alcohols. The figure shows schematically the conversion of racemic amphetamine with enantiomerically pure ( R ) -Mosher acid. While the enantiomers ( R ) - and ( S ) -amphetamine show identical NMR spectra, the diastereomers ( RS ) and ( RR ) differ from one another in the NMR. Depending on the response, shifts of up to 47 Hertz are possible.
( R ) - or ( S ) -α-methoxy-α- (trifluoromethyl) phenylacetic acid is, like its esters and amides , insensitive to the risk of racemization, since there is no α-hydrogen atom next to the carbonyl group and thus no enol can form.
Other chiral derivatization reagents
While many chiral derivatization reagents are fluorinated, there are also approaches to use 31 P spectroscopy to study enantiomers. Reiner et al. investigated a chlorophosphine based on enantiomerically pure BINOL . In 31 P-NMR, derivatized chiral alcohols and amines can be distinguished with the reagent. The Δδ values are up to 6.7 ppm .
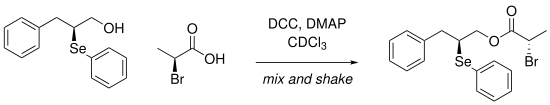
Another approach is pursued by Gonçalves et al., Who use NMR spectra of 77 Se to determine ee values . To do this, they developed a mix and shake method to react carboxylic acids directly in the NMR tube with an enantiomerically pure α-seleno alcohol and then to investigate them. Here are DCC and DMAP as reagents for Estersynthese used. The Δδ values in the 77 Se-NMR are up to 211 ppm.
Individual evidence
- ^ JL Mateos and DJ Cram : Studies in Stereochemistry. XXXI. Conformation, Configuration and Physical Properties of Open-chain Diastereomers . In: J. Am. Chem. Soc. . 81, No. 11, 1959, pp. 2756-2762. doi : 10.1021 / ja01520a037 .
- ↑ JA Dale, DL Dull and HS Mosher : α-Methoxy-α-trifluoromethylphenylacetic acid, a versatile reagent for the determination of enantiomeric composition of alcohols and amines . In: J. Org. Chem. . 34, No. 9, 1969, pp. 2543-2549. doi : 10.1021 / jo01261a013 .
- ↑ D. Parker: NMR determination of enantiomeric purity . In: Chem Rev.. . 91, No. 7, 1991, pp. 1441-1457. doi : 10.1021 / cr00007a009 .
- ↑ Thomas Reiner, Frederik N. Naraschewski, Jörg Eppinger: 31 P NMR assays for rapid determination of enantiomeric excess in catalytic hydrosilylations and transfer hydrogenations . In: Tetrahedron: Asymmetry . tape 20 , no. 3 , February 2009, p. 362-367 , doi : 10.1016 / j.tetasy.2009.01.022 .
- ↑ Jeiely G. Ferreira, Simone MC Gonçalves: enantiomeric excess detection with (S) -3-phenyl-2- (selenophenyl) propane-1-ol derivatizing agent via mix and shake 77Se NMR . In: Journal of the Brazilian Chemical Society . tape 21 , no. 11 , 2010, p. 2023-2026 , doi : 10.1590 / S0103-50532010001100002 .