Corey-Nicolaou macrolactonization
The Corey Nicolaou macrolactonization (aka Corey Nicolaou double activation) is a name reaction of organic chemistry , by Elias James Corey and K. C. Nicolaou was published 1974th This synthesis is used to produce macrocyclic lactones ( macrolides ).
Overview reaction
With the help of the Corey-Nicolaou macrolactonization, a macrolide can be produced from an ω-hydroxycarboxylic acid and using 2,2'-dipyridyl disulfide and triphenylphosphine (PPh 3 ) .
General
Corey-Nicolaou macrolactonization makes it possible to synthesize medium-sized to very large lactones . With the help of this reaction the production of 7- to 48-membered rings has been possible so far. Xylenes , toluene or benzene are used as solvents . Most of the reaction takes place at room temperature or at 25 ° C. and over a longer period of time. Because of the mild reaction conditions, this reaction is also suitable for polyfunctional substances.
Reaction mechanism
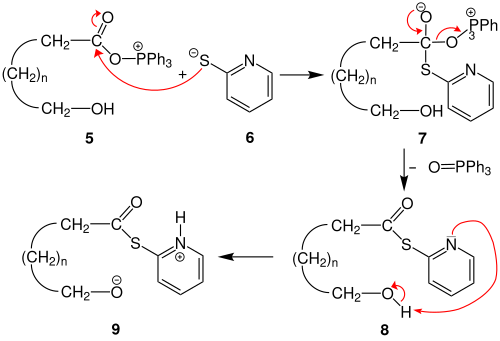
The reaction mechanism of the Corey-Nicolaou macrolactonization could be as follows. In the first step, the lone pair of electrons of the triphenylphosphine (PPh 3 ) attacks a sulfur atom of the 2,2'-dipyridyl disulfide 1 nucleophilically . The bond between the sulfur atoms of the 2,2'-dipyridyl disulfide breaks. The anion 2 and the cation 3 are formed .
In the next step, the carboxy group of the ω-hydroxycarboxylic acid 4 is deprotonated by the lone pair of electrons on the nitrogen of anion 2 . This forms 2-pyridinthione. The carboxylate group attacks the phosphonium group of cation 3 . The cation 5 and the anion 6 are formed .
The anion 6 attacks the acyl group of the cation 5 nucleophilically . The tetrahedral intermediate 7 is formed . With reformation of the acyl group to the thioester 8 is triphenylphosphine oxide removed. The nitrogen lone pair of electrons deprotonates the ω-hydroxy group. The zwitterion 9 is created .
By an intramolecular nucleophilic attack of the acyl group through the alcoholate and simultaneous deprotonation of Pyridiniumrests generated intermediate 10 . With regression of the acyl group and elimination of 2-pyridinthione, the macrolide 11 is formed .
application
The Corey-Nicolaou macrolactonization is one of the most efficient reactions of its kind. The yield varies depending on the ring size to be achieved. With a few exceptions, 60–80% of the product can be isolated up to a ring size of 16 members. Exceptions can be traced back to unfavorable ring stresses . It is particularly suitable for the synthesis of natural substances and is used in many total syntheses .
Individual evidence
- ↑ a b c Jie Jack Li: Name reactions. A collection of detailed reaction mechanisms . 3. Edition. Springer-Verlag, Berlin 2006, ISBN 978-3-540-30030-4 , pp. 164-165 , doi : 10.1007 / 3-540-30031-7 .
- ↑ a b c d E. J. Corey, Kyriacos C. Nicolaou: An Efficient and Mild Lactonization Method for the Synthesis of Macrolides . In: Journal of the American Chemical Society . tape 96 , no. 17 , 1974, p. 5614–5616 , doi : 10.1021 / ja00824a073 .
- ↑ EJCorey, Daniel J Brunelle: New reagents for the conversion of hydroxy acids to macrolactones by the double activation method . In: Tetrahedron . tape 17 , no. 38 , 1976, p. 3409-3412 , doi : 10.1016 / S0040-4039 (00) 93057-0 .
- ↑ KC Nicolaou: Synthesis of macrolides . In: Tetrahedron . tape 33 , no. 7 , 1977, pp. 683-710 , doi : 10.1016 / 0040-4020 (77) 80180-4 .
- ↑ A. Parenty, X. Moreau, J.-M. Campagne: Macrolactonizations in the Total Synthesis of Natural Products . In: Chemical Reviews . tape 106 , no. 3 , 2006, p. 911-939 , doi : 10.1021 / cr0301402 .