Cyclopropyl group
In chemistry, a cyclopropyl group is a substituent which is derived from the alicyclic hydrocarbon cyclopropane . With the empirical formula C 3 H 5 , it is the smallest cycloalkyl substituent.
properties
In the homologous series of cycloalkyl substituents, the cyclopropyl group stands out due to its special properties, since it contains the bent bonds present in cyclopropane . Its character is similar to the "unsaturated" vinyl group ; it can conjugate with neighboring double bond systems (π electron systems) as a π donor. For this, however, a defined spatial position of the three-membered ring in the molecule ( conformation ) is necessary (see below). Evidence and strong evidence were provided by spectroscopic investigations of cyclopropyl-substituted compounds, e.g. B. cyclopropylbenzene or cyclopropanecarbaldehyde , but above all cyclopropylmethyl derivatives, if carbocations are formed from them as intermediates.
Examples
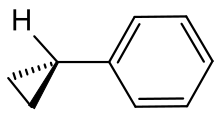
Cyclopropylbenzene
This hydrocarbon consists of two rigid rings, but a rotation around the linking CC bond is possible. An NMR study determined a value of 2.0 kcal mol −1 (8.4 kJ mol −1 ) for the rotation barrier. The energy minimum corresponds to a conformation in which the plane of the benzene ring (phenyl ring) in the projection symmetrically cuts the opposite CC bond of the cyclopropane ring (atoms C-2 and C-3) in half. This conformation was called "bisected" in English; the tertiary hydrogen atom of the cyclopropyl substituent then lies in the plane defined by the benzene ring. On the basis of the Walsh model , it has been argued that in this arrangement the interaction between a HOMO of the cyclopropyl group and a LUMO of the phenyl ring reaches a maximum, and therefore the conjugation is optimal. This model can also be used to explain the different C — C bond lengths of the cyclopropyl group: the two vicinal bonds are longer (151.4 pm) than the distal bond (150.7 pm).
Cyclopropanecarbaldehyde
Two energy minima have been identified here. Both conformations are "bisectic", differ only in the position of the carbonyl oxygen atom. In one case the O atom points towards the three-membered ring, in the other the H atom; both times the conjugation should be optimal.
Solvolysis of 4-cyclopropylcumyl chloride
When 2-chloro-2-phenylpropane (“cumyl chloride”) reacts with water, which has been mixed with acetone to improve solubility, a carbocation is formed as a reactive intermediate , with the chloride dissociating . A cyclopropyl substituent in the para position accelerates this hydrolysis (“solvolysis”) at 25 ° C by over one hundred and fifty times, in contrast to the cyclobutyl, cyclopentyl and cyclohexyl radicals, which only react about twenty times faster.
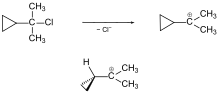
Cyclopropylmethyl compounds
The solvolysis of 2-cyclopropyl-2-chloropropane is faster than that of 2-chloro-2-methylpropane ( tert-butyl chloride), in which the tert- butylium ion occurs as an intermediate.
In the case of the cyclopropyl compound, a cyclopropylmethyl cation is formed (previously also known as the cyclopropylcarbinyl cation). This could even be investigated in the strongly ionizing medium SbF 5 -SO 2 ClF by NMR spectroscopy.
The most persistent (“most stable”) known carbocation in this series is the tricyclopropylmethyl cation.
literature
- Zvi Rappoport (Ed.), The Chemistry of the Cyclopropyl Group, Volume 1, Wiley, Chichester u. a. O., 1987. Volume 2, Wiley, Chichester 1995. ISBN 0-471-94074-7
Individual evidence
- ^ William JE Parr, Ted Schaefer: A proton magnetic resonance investigation of the preferred conformation and the barrier to internal rotation of phenylcyclopropane . In: J. Amer. Chem. Soc. , 99 (4), pp. 1033-1035. (1977), doi : 10.1021 / ja00446a010 .
- ↑ a b Quang Shen, Christopher Wells, Marit Traetteberg, Robert K. Bohn, Amanda Willis, Joseph Knee: Molecular Structure and Conformation of Cyclopropylbenzene As Determined by ab Initio Molecular Orbital Calculations, Pulsed-Jet Fourier Transform Microwave Spectroscopic, and Gas-Phase Electron Diffraction Investigations . In: J. Org. Chem. 66 (17), pp. 5840-5845 (2001), doi : 10.1021 / jo010293u .
- ↑ HN Volltrauer, RH Schwendeman: Microwave Spectra, Dipole Moments, and Torsional Potential Constants of cis‐ and trans ‐ Cyclopropanecarboxaldehyde . In: J. Chem. Phys. 54 (1971), p. 260, doi : 10.1063 / 1.1674602 .
- ↑ HC Brown, JD Cleveland, J. Org. Chem. 41, p. 1792 (1976).
- ↑ HC Brown, BG Gnedin, K. Takeuchi, EN Peters, JACS 97, p. 610 (1975).
- ↑ DP Kelly, HC Brown, JACS 97, p. 3879 (1975).
- ↑ GA Olah, CL Jeuell, DP Kelly, RD Porter, JACS 94, p. 146 (1972).
- ↑ Norman C. Deno, Herman G. Richey, Jane S. Liu, James D. Hodge, John J. Houser, Max J. Wisotsky. Physical Properties of the Tricyclopropylmethyl Cation In: Journal of the American Chemical Society 84, pp. 2016-2017 (1962).
- ↑ Harold Hart, Paul A. Law. The Solvolysis of Tricyclopropylcarbinyl Benzoate , In: Journal of the American Chemical Society 86, pp. 1957-1959 (1964).