Cyclopropanecarbaldehyde
| Structural formula | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
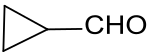
|
||||||||||||||||
| General | ||||||||||||||||
| Surname | Cyclopropanecarbaldehyde | |||||||||||||||
| other names |
|
|||||||||||||||
| Molecular formula | C 4 H 6 O | |||||||||||||||
| Brief description |
colorless to yellow liquid |
|||||||||||||||
| External identifiers / databases | ||||||||||||||||
|
||||||||||||||||
| properties | ||||||||||||||||
| Molar mass | 70.09 g mol −1 | |||||||||||||||
| Physical state |
liquid |
|||||||||||||||
| density | ||||||||||||||||
| boiling point | ||||||||||||||||
| solubility |
Completely soluble in water, ethanol and diethyl ether as well as in dichloromethane |
|||||||||||||||
| Refractive index |
1.4298 (20 ° C) |
|||||||||||||||
| safety instructions | ||||||||||||||||
|
||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . Refractive index: Na-D line , 20 ° C | ||||||||||||||||
Cyclopropanecarbaldehyde is the smallest cycloalkane that carries a formyl group that is directly linked to the cyclopropyl group . The compound is a molecule module ( building block ) for the dinitroaniline - herbicide profluralin and cyclopropylacetylene, an important intermediate ( intermediate ) for the HIV -Therapeutikum efavirenz .
Manufacturing
The oxidation of cyclopropylmethanol with aqueous ammonium cerium (IV) nitrate (NH 4 ) 2 [Ce (NO 3 ) 6 ] gives cyclopropanecarboxaldehyde in 64% yield.
Even when shaking with potassium permanganate attached to aluminum silicate , cyclopropanemethanol produces CPCA in 89% yield.
The by acyloin condensation of diethyl succinate easily accessible 2-Hydroxycyclobutanon can with lithium aluminum hydride can be hydrogenated to 1,2-cyclobutanediol, which with boron trifluoride -dibutyletherat at 230 ° C with elimination of water and ring contraction in 65-80% yield to cyclopropanecarboxaldehyde responding.
Thermal rearrangement of 2,3-dihydrofuran at temperatures from 200 to 300 ° C., elevated pressure (4.5 bar) and in the presence of an aluminum oxide catalyst produces a mixture of approx. 90% cyclopropanecarbaldehyde (through ring narrowing) and which can not be separated by distillation approx. 10% crotonaldehyde with boiling point 102.4 ° C. through ring opening .
The mixture of substances is separated by catalytic hydrogenation of the more reactive crotonaldehyde to butanal (bp. 75 ° C.) and subsequent distillation.
An alternative is to react the mixture with a secondary amine, e.g. B. dicyclohexylamine , where with crotonaldehyde mainly higher boilers are formed, which remain in the sump when the pure CPCA is separated off by distillation.
properties
Cyclopropanecarboxaldehyde is a clear, colorless to yellow, smelly liquid that dissolves completely with water and with many organic solvents, such as B. ethanol, diethyl ether or methylene chloride mixes.
Applications
By reductive amination of CPCA with propylamine and hydrogen in the presence of platinum as a catalyst, cyclopropylmethyl-N-propylamine is produced as the precursor of the herbicide profluralin (Tolban R ).
The multi-stage synthesis of cyclopropylacetylene (ethynylcyclopropane) as a molecular building block for the virustatic efavirenz is based on cyclopropane carbaldehyde, which reacts with malonic acid to form cyclopropylacrylic acid. The bromination with N-bromosuccinimide gives cyclopropyl vinyl bromide which is dehydrobrominated with potassium tert-butoxide in dimethyl sulfoxide in 80% yield to cyclopropylacetylene.
A three-stage alternative cyclopropane acetylene synthesis, starting from CPCA, proceeds via the addition of trichloroacetic acid to α- (trichloromethyl) cyclopropane methanol, which is reacted with para-toluenesulfonic acid chloride to form the tosylate . From this, chloride, tosylate and hydrogen chloride are split off with methyllithium to form ethynylcyclopropane.
The method is also suitable for the preparation of 1- alkynes from the corresponding aldehydes in good to very good yields.
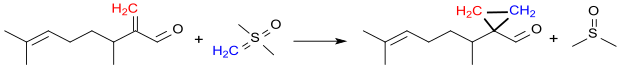
Some alkenyl-substituted cyclopropanecarboxaldehydes, such as e.g. B. the compound 1- (1,5-dimethyl-4-hexenyl) -cyclopropanecarboxaldehyde, are suitable as perfume components due to their flowery smell. Such substituted cyclopropanecarbaldehydes are not obtained from CPCA, but are obtained by Johnson-Corey-Chaykovsky reaction e.g. B. the cyclopropanation of 3,7-dimethyl-2-methylene-oct-6-enal with dimethyloxosulfonium methylide - accessible from trimethyloxosulfonium iodide with sodium hydride analogously to the instructions.
Individual evidence
- ↑ a b Datasheet Cyclopropanecarboxaldehyde at AlfaAesar, accessed on March 30, 2018 ( PDF )(JavaScript required) .
- ↑ a b Entry on Cyclopropanecarboxaldehyde at TCI Europe, accessed on March 30, 2018.
- ^ A b c William M. Haynes: CRC Handbook of Chemistry and Physics, 97th Edition . CRC Press, Boca Raton, FL, USA 2017, ISBN 978-1-4987-5429-3 , pp. 3-138 .
- ↑ a b c data sheet Cyclopropanecarboxaldehyde 98% at Sigma-Aldrich , accessed on March 30, 2018 ( PDF ).
- ↑ a b J.P. Barnier, J. Champion, JM Conia: Cyclopropanecarboxaldehyde In: Organic Syntheses . 60, 1981, p. 25, doi : 10.15227 / orgsyn.060.0025 ; Coll. Vol. 7, 1990, p. 129 ( PDF ).
- ↑ LB Young, WS Trahanovsky: Cerium (IV) oxidation of organic compounds. III. Preparation of cyclopropanecarbaldehydes from cyclopropanemethanol . In: J. Org. Chem. Band 32 , no. 7 , 1967, p. 2349-2350 , doi : 10.1021 / jo01282a058 .
- ↑ J.-D. Lou, C.-L. Gao, L. Li. Z.-G. Fang: An Efficient Selective Oxidation of Alcohols with Potassium Permanganate Adsorbed on Aluminum Silicate under Solvent-free Conditions and Shaking . In: monthly Chem. Band 137 , no. 8 , 2006, p. 1071-1074 , doi : 10.1007 / s00706-006-0506-0 .
- ↑ JJ Bloomfield, JM Nelke: Acyloin condensation in which trichloromethylsilane is used as a trapping agent: 1,2-bis (trimethylsilyloxy) cyclobutene and 2-hydroxycyclobutanone In: Organic Syntheses . 75, 1977, p. 1, doi : 10.15227 / orgsyn.057.0001 ; Coll. Vol. 6, 1988, p. 167 ( PDF ).
- ↑ Patent US5633410 : Process for the conversion of 2,3-dihydrofuran to cyclopropanecarboxaldehyde. Applied August 30, 1996 , published May 27, 1997 , Applicant: Eastman Chemical Co., Inventor: S. Liang, TW Price.
- ↑ Patent US5471003 : Purification of cyclopropanecarboxaldehyde. Applied on November 28, 1994 , published on November 28, 1995 , applicant: Eastman Chemical Co., inventor: S. Liang.
- ↑ Patent US6353140B1 : Process for the purification of cyclopropanecarboxaldehyde. Applied June 8, 2001 , published March 5, 2002 , Applicant: Eastman Chemical Co., Inventor: SN Falling, SE Large, RJ Maleski.
- ↑ Patent US4275238 : Process for the preparation of cyclopropylmethyl-N-propylamine. Applied January 10, 1980 , published June 23, 1981 , Applicant: Ciba-Geigy Corp., Inventor: HE Petree, JB Nabors.
- ↑ Patent US6297410B1 : Process for the preparation of cyclopropylacetylene. Filed January 12, 2000 , published October 2, 2001 , Applicant: DuPont Pharmaceutical Co., Inventor: JM Fortunak, Z. Wang, J. Yin.
- Jump up ↑ Z. Wang, S. Campagna, K. Yang, G. Xu, ME Pierce, JM Fortunak, PN Confalone: A practical preparation of terminal alkynes from aldehydes . In: J. Org. Chem. Band 65 , no. 6 , 2000, pp. 1889-1891 , doi : 10.1021 / jo9916582 .
- ↑ Patent US7087796B1 : Cyclopropane carboxaldehydes and their use in perfume compositions. Filed June 16, 2005 , published August 8, 2006 , Applicant: International Flavors & Fragrances Inc., Inventors: APS Narula, EM Arruda, FT Schiet.
- ↑ EJ Corey, M. Chaykovsky: Methylenecyclohexane oxide In: Organic Syntheses . 49, 1969, p. 78, doi : 10.15227 / orgsyn.049.0078 ; Coll. Vol. 5, 1973, p. 755 ( PDF ).