para -Toluenesulfonic acid chloride
| Structural formula | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|

|
||||||||||||||||
| General | ||||||||||||||||
| Surname | para -Toluenesulfonic acid chloride | |||||||||||||||
| other names |
|
|||||||||||||||
| Molecular formula | C 7 H 7 ClO 2 S | |||||||||||||||
| Brief description |
light gray solid with a characteristic odor |
|||||||||||||||
| External identifiers / databases | ||||||||||||||||
|
||||||||||||||||
| properties | ||||||||||||||||
| Molar mass | 190.64 g mol −1 | |||||||||||||||
| Physical state |
firmly |
|||||||||||||||
| density |
1.49 g cm −3 |
|||||||||||||||
| Melting point |
67 ° C |
|||||||||||||||
| boiling point |
135 ° C (13 hPa) |
|||||||||||||||
| Vapor pressure |
0.16 Pa at 25 ° C |
|||||||||||||||
| solubility |
Decomposes with water |
|||||||||||||||
| safety instructions | ||||||||||||||||
|
||||||||||||||||
| Toxicological data | ||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||
para -Toluenesulfonic acid chloride is a chemical compound from the group of sulfonic acids . It is the acid chloride of para- toluenesulfonic acid and is colloquially often referred to as tosyl chloride, written TsCl. The abbreviation “Ts” stands for a tosyl group .
presentation
The compound can be prepared from toluene by a reaction similar to Friedel-Crafts alkylation . For this purpose, toluene is reacted with sulfuryl chloride and aluminum chloride as a catalyst .
Another possibility is the conversion of para- methylaniline in a Sandmeyer -like reaction. To do this, the amine is first diazotized with sodium nitrite . The resulting diazonium salt is then reacted with sulfur dioxide in the presence of copper (I) chloride and magnesium chloride .
Tosyl chloride is a waste product from saccharine production. In addition to the o- toluenesulphonyl chloride required for this, the p -toluenesulphonyl chloride is also produced here, which is sold as a fine chemical after separation from one another.
properties
para -Toluenesulfonic acid chloride is a corrosive solid with a characteristic peculiar odor, which melts at 67 ° C and boils at 135 ° C at a pressure of 13 hPa. It decomposes from a temperature of 220 ° C. A DSC measurement shows a strongly exothermic decomposition reaction from 249 ° C with an exothermicity of −451 kJ kg −1 or −86 kJ mol −1 .
use
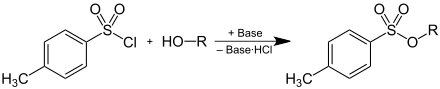
Tosylates can be produced by deprotonating an alcohol and then reacting with p -toluenesulphonic acid chloride, whereby one equivalent of hydrogen chloride , which is bound as a hydrochloride by a base , is formed:
Due to their property as a leaving group, tosylates are used as intermediates in preparative organic chemistry . By converting alcohols into tosylates, the poor leaving group HO - is converted into a good leaving group, which enables substitution reactions at this position on the carbon structure. The anion (= tosylate ) of p -toluenesulfonic acid emerges as a leaving group .
literature
- Reinhard Brückner : reaction mechanisms . 3rd edition, Spektrum Akademischer Verlag, Munich 2004, ISBN 3-8274-1579-9 .
- Author collective: Organikum . 22nd edition. Wiley-VCH, 2004, ISBN 978-3-527-31148-4 .
Individual evidence
- ↑ a b c d e f g h Entry on toluene-4-sulfonyl chloride in the GESTIS substance database of the IFA , accessed on January 10, 2017(JavaScript required) .
- ↑ OECD : Screening Information Dataset (SIDS) Initial Assessment Report (SIAR) for Benzenesulfonyl chloride, 4-methyl- , accessed on June 15, 2017.
- ↑ Data sheet para-toluenesulfonic acid chloride (PDF) from Merck , accessed on January 18, 2011.
- ↑ Paula Yurkanis Bruice: Organic Chemistry , Pearson Education Inc., 2004, 4th edition, p 444, ISBN 0-13-121730-5 .
- ↑ A. Töhl, O. Eberhard: About the action of sulfuryl chloride on aromatic hydrocarbons , in: Chem. Ber. 1893 , 26 , 2940-2945; doi : 10.1002 / cber.189302603118 .
- ↑ H. Meerwein, G. Dittmar, R. Göllner, K. Hafner, F. Mensch, O. Steinfort: Investigations on aromatic diazo compounds, II. Process for the production of aromatic sulfonic acid chlorides, A new modification of the Sandmeyer's reaction , in: Chem. Ber . 1957 , 90 , 841-852; doi : 10.1002 / cber.19570900602 .
- ^ H. Beyer, W. Walter: Textbook of organic chemistry, S. Hirzel Verlag, Stuttgart 1981, 19th edition, p. 514; ISBN 3-7776-0356-2 .
- ↑ Sperry, JB; Minteer, CJ; Tao, J .; Johnson, R .; Duzguner, R .; Hawksworth, M .; Oke, S .; Richardson, PF; Barnhart, R .; Bill, DR; Giusto, RA; Weaver, JD: Thermal Stability Assessment of Peptide Coupling Reagents Commonly Used in Pharmaceutical Manufacturing in Org. Process Res. Dev. 22 (2018) 1262-1275, doi : 10.1021 / acs.oprd.8b00193 .