Cyclopropylmethanol
| Structural formula | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|

|
||||||||||||||||
| General | ||||||||||||||||
| Surname | Cyclopropylmethanol | |||||||||||||||
| other names |
|
|||||||||||||||
| Molecular formula | C 4 H 8 O | |||||||||||||||
| Brief description |
clear, colorless to light yellow liquid |
|||||||||||||||
| External identifiers / databases | ||||||||||||||||
|
||||||||||||||||
| properties | ||||||||||||||||
| Molar mass | 72.11 g mol −1 | |||||||||||||||
| Physical state |
liquid |
|||||||||||||||
| density | ||||||||||||||||
| Melting point |
|
|||||||||||||||
| boiling point | ||||||||||||||||
| Vapor pressure |
5.4 hPa at 20 ° C |
|||||||||||||||
| solubility |
Completely miscible with water and soluble in many organic solvents |
|||||||||||||||
| Refractive index |
1.4320 - 1.4330 (20 ° C) |
|||||||||||||||
| safety instructions | ||||||||||||||||
|
||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . Refractive index: Na-D line , 20 ° C | ||||||||||||||||
Cyclopropylmethanol is the smallest cycloalkane that carries a hydroxymethyl group that is directly linked to the cyclopropyl group . CPMO differs from other functional cyclopropanes in its pronounced tendency to react under more drastic reaction conditions by ring expansion to give cyclobutane derivatives and / or ring opening to give linear n -butane derivatives . The compound is the starting material for active pharmaceutical ingredients and pesticides .
Manufacturing
Catalytic hydrogenation of cyclopropanecarbaldehyde in the presence of Raney nickel or Raney cobalt produces cyclopropylcarbinol in yields of up to> 90% - in addition to n- butanol through splitting of the cyclopropane ring.
The reduction of cyclopropanecarboxylic acid with lithium aluminum hydride LiAlH 4 yields CPMO, as does the hydrogenation of its esters, e.g. B. on a zinc oxide contact in almost quantitative yield,
A variant of the Simmons-Smith reaction with metallic lanthanum and diiodomethane converts allyl alcohol into cyclopropane methanol with a yield of 67% in a cyclopropanation reaction.
properties
Cyclopropanemethanol is a clear, colorless liquid that dissolves completely with water and with many organic solvents, such as e.g. B. dimethylformamide , dichloromethane , or tetrahydrofuran mixes.
Applications
Synthesis of cyclopropylmethyl halides
The easiest way from cyclopropylmethanol to cyclopropylmethyl bromide would be the reaction with aqueous hydrobromic acid . However, in 82% overall yield, even at low temperatures (5 to 10 ° C), a mixture of 56% cyclopropylmethyl bromide (I) (bp 106 ° C) and 37% cyclobutyl bromide (II) (bp 108 ° ) is difficult to separate by distillation C) and 7% 4-bromo-1-butene (III) (bp 100 ° C).
Mild process variants, such as B. with dimethyl sulfide / N -bromosuccinimide complex or triphenylphosphite / bromine do produce significantly fewer by-products due to ring expansion or cleavage, but also no significantly better yields of cyclopropylmethyl bromide (67 or 73%) in addition to unreacted starting material. The reaction with triphenylphosphine / bromine also offers no advantages with 72% yield, since the dreaded triphenylphosphine oxide , which is often not completely separable, is also formed as a by-product. Complex formation with zinc chloride has recently been proposed for its separation from polar solvents . Highly pure cyclopropylmethyl bromide, as it is for synthesis variants to pharmaceutical active ingredients, such as. B. the benzodiazepine prazepam , the beta blocker betaxolol and the opioid antagonist naltrexone , is accessible by converting CPMO in dimethylformamide with triphenylphosphine and an excess of bromine.
The chloromethylcyclopropane, which is much more stable against rearrangement, is formed in almost quantitative yield in the reaction of cyclopropylcarbinol with dimethyl sulfide / N -chlorosuccinimide , while the mild chlorinating agent hexachloroacetone / triphenylphosphane produces cyclopropylmethyl chloride in 87% yield.
The more drastic reaction with concentrated hydrochloric acid under reflux (100 ° C.) also leads to ring expansion and the formation of cyclobutanol in 57% yield.
In the same way, cyclopropylcarbinol reacts with aqueous hydrofluoric acid in 98% yield to give cyclobutyl fluoride in addition to 4-fluoro-1-butene (2%), i. H. Cyclopropylmethyl fluoride is not formed at all.
The preparation of cyclopropylmethyl fluoride, however, succeeds in the reaction of cyclopropane methanol with diethylaminosulfur trifluoride DAST at −50 ° C.
Cyclopropylmethyliodide can be obtained by halogen exchange from CPMO with sodium iodide in acetone with a yield of 57%.
Other derivatives of cyclopropylmethanol
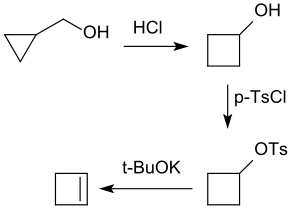
Cyclopropylmethanol reacts in a one-pot reaction with hydrochloric acid with ring expansion to give cyclobutanol and can then be oxidized with chromium (VI) oxide with yields of approx. 35% to give cyclobutanone .
The cyclobutanol produced by the acid-catalyzed rearrangement of cyclopropylmethanol reacts smoothly with para- toluenesulfonic acid chloride to form the tosylate . Heating with potassium tert -butanolate in dimethyl sulfoxide DMSO gives cyclobutene in yields of 70 - 84%.
Cyclopropylmethanol is oxidized by ammonium cerium (IV) nitrate in aqueous solution in 64% yield to cyclopropanecarbaldehyde.
Individual evidence
- ↑ a b c d data sheet cyclopropanemethanol at AlfaAesar, accessed on February 8, 2018 ( PDF )(JavaScript required) .
- ↑ a b c d e data sheet cyclopropanemethanol from Sigma-Aldrich , accessed on February 8, 2018 ( PDF ).
- ^ William M. Haynes: CRC Handbook of Chemistry and Physics, 97th Edition . CRC Press, Boca Raton, FL, USA 2017, ISBN 978-1-4987-5429-3 , pp. 3-138 .
- ↑ a b c d Patent US6118032 : Process for the production of cyclopropylmethyl halides. Filed August 3, 1999 , published September 12, 2000 , Applicant: Eastman Chemical Co., Inventors: DJ Bayston, RM Scott, JM Lovell, LA White.
- ↑ Entry on cyclopropanemethanol at TCI Europe, accessed on February 8, 2018.
- ↑ Patent US5475151 : Process for the preparation of cyclopropylmethanol. Applied November 28, 1994 , published December 12, 1995 , Applicant: Eastman Chemical Co., Inventor: S. Liang, TW Price.
- ↑ Patent US3454575 : Quaternary 5-ammonium-methyl-4-amino-2-cycloaliphatylpyrimidine salts. Applied on March 6, 1967 , published July 8, 1969 , applicant: Ciba Corp., inventor: RH Mizzoni, G. de Stevens.
- ↑ Patent EP0728722A1 : Process for the production of hydroxymethyl-cyclopropane. Applied on February 8, 1996 , published on August 28, 1996 , Applicant: Bayer AG, Inventors: L. Frohn, R. Langer, G. Darsow, E. Zirngiebl, J.-D. Jentsch, B. Pennemann, C. Tiburtius.
- ↑ Y. Nishiyama, H. Tanimizu, T. Tomita: Lanthanum metal-assisted cyclopropanation of alkenes with gem-dihalogenalkanes . In: Tetrahedron Lett. tape 48 , no. 36 , 2007, p. 6405-6407 , doi : 10.101016 / j.tetlet.2007.06.0168j .
- ↑ Patent US6191300B1 : Process for the preparation of cyclopropylacetonitrile. Filed April 16, 1999 , published February 20, 2001 , applicant: Eastman Chemical Co., inventor: JA Hyatt.
- ↑ Patent US20160355452A1 : Method for producing (bromomethyl) cyclopropane and (bromomethyl) cyclobutane. Registered on December 1, 2014 , published on December 8, 2016 , applicant: Melchior Material and Life Science France, inventor: O. Guerret.
- ↑ a b R.T. Hrubiec, MB Smith: Regioselective route to sterically hindered cyclopropylcarbinyl halides . In: J. Org. Chem. Band 49 , no. 3 , 1984, pp. 431-435 , doi : 10.1021 / jo00177a008 .
- ↑ DC Batesky, MJ Goldfogel, DJ Weix: Removal of Triphenylphosphine Oxide by Precipitation with Zinc Chloride in Polar Solvents . In: J. Org. Chem. Band 82 , no. 19 , 2017, p. 9931-9936 , doi : 10.1021 / acs.joc.7b00459 .
- ↑ Patent US6008420 : Process for the production of halogen methyl cyclopropanes and highly pure halogen methyl cyclopropanes. Registered on August 20, 1998 , published on December 28, 1999 , applicant: Bayer AG, inventor: J. Komoschinski, R. Gehring.
- ↑ RM Magid: Triphenylphosphine-Hexachloroacetone . In: e-EROS Encyclopedia of Reagents for Organic Synthesis . 2001, doi : 10.1002 / 047084289X.rt373 .
- ↑ a b J. Salaün, A. Fadel: Cyclobutene In: Organic Syntheses . 64, 1986, p. 50, doi : 10.15227 / orgsyn.064.0050 ; Coll. Vol. 7, 1990, p. 117 ( PDF ).
- ↑ M. Hanack, H. Eggensperger: Organic fluorine compounds, VIII Conversion of cyclopropylcarbinol, cyclobutanol and cyclobutene with hydrogen fluoride. Preparation of fluoromethylcyclopropyl ketone . In: Chem. Ber. tape 96 , no. 5 , 1963, pp. 1341-1349 , doi : 10.1002 / cber.19630960525 .
- ^ JR Durig, Z. Yu, C. Zheng, GA Guirgis: Conformational Studies of Fluoromethylcyclopropane from Temperature-Dependent FT-IR Spectra of Xenon Solutions and Ab Initio Calculations . In: J. Phys. Chem. A . tape 108 , no. 25 , 2004, pp. 5353-5364 , doi : 10.1021 / jp0401168 .
- ↑ DH see, CJ Michejda: Acid-catalyzed decomposition of trialkyltriazenes: Protected alkyldiazonium ions . In: J. Am. Chem. Soc. tape 103 , no. 2 , 1981, p. 442-445 , doi : 10.1021 / ja00392a032 .
- ↑ M. Krumpolc, J. Rocek: Cyclobutanone In: Organic Syntheses . 60, 1981, p. 20, doi : 10.15227 / orgsyn.060.0020 ; Coll. Vol. 7, 1990, p. 114 ( PDF ).
- ↑ LB Young, WS Trahanovsky: Cerium (IV) oxidation of organic compounds. III. Preparation of cyclopropanecarbaldehydes from cyclopropanemethanol . In: J. Org. Chem. Band 32 , no. 7 , 1967, p. 2349-2350 , doi : 10.1021 / jo01282a058 .