Prazepam
| Structural formula | ||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
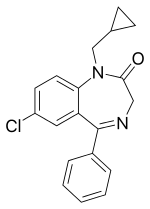
|
||||||||||||||||||||||
| General | ||||||||||||||||||||||
| Surname | Prazepam | |||||||||||||||||||||
| other names |
7-chloro-1- (cyclopropylmethyl) -1,3-dihydro-5-phenyl-2 H -1,4-benzodiazepin-2-one |
|||||||||||||||||||||
| Molecular formula | C 19 H 17 ClN 2 O | |||||||||||||||||||||
| Brief description |
white solid with a characteristic odor |
|||||||||||||||||||||
| External identifiers / databases | ||||||||||||||||||||||
|
||||||||||||||||||||||
| Drug information | ||||||||||||||||||||||
| ATC code | ||||||||||||||||||||||
| properties | ||||||||||||||||||||||
| Molar mass | 324.80 g mol −1 | |||||||||||||||||||||
| Physical state |
firmly |
|||||||||||||||||||||
| Melting point |
147-148 ° C |
|||||||||||||||||||||
| safety instructions | ||||||||||||||||||||||
|
||||||||||||||||||||||
| Toxicological data | ||||||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||||||||
Prazepam is a drug from the group of benzodiazepines with calming, anxiety-relieving , sleep-promoting , as well as centrally muscle-relaxing and anticonvulsant effects.
It is one of the long-acting benzodiazepines.
Pharmacodynamics
Like all representatives of the benzodiazepine group, prazepam acts on the GABA A receptor by increasing the affinity of the inhibitory neurotransmitter GABA and finally by increasing the influx of chloride ions, which leads to a lower excitability of the neuron membrane due to hyperpolarization and short-circuiting of EPSPs . In particular, the muscle-relaxing effect of GABA is enhanced. The inhibition constant for prazepam is 110 nM −1 , for nordazepam it is 10 nM −1 .
Pharmacokinetics
After ingestion of prazepam, the cytochrome P450 CYP3A4 first metabolizes it to nordazepam (main metabolite) and then to oxazepam . Prazepam is therefore a prodrug . After glucuronidation, it is excreted via the kidneys. The maximum plasma concentration is reached after 0.5 to 4 hours, for the metabolite nordazepam after two to eight hours. The mean plasma half-life is about 1.3 hours for prazepam and 50 to 80 hours for the main metabolite northazepam, although strong individual fluctuations are possible.
indication
Prazepam is indicated for the symptomatic treatment of acute and chronic states of tension, excitement and anxiety. Prazepam should only be used as a sleep aid if benzodiazepine effects are desired on the following day.
Side effects
Like all other drugs from the benzodiazepine group, prazepam has side effects such as CNS depression (depression of the sympathetic nervous system ), ataxia ( cerebellar disorder), visual disturbances (impaired vision), amnesia , headaches and motor slowdowns, lethargy . With long-term use in high doses, typical neurological patterns such as deviations in the coordination correction of movements occur. These indicate a disruption of the feedback circuit between the cerebellum and the nucleus ruber (clear indications in neurology are finger-nose tests with deviation). As with all benzodiazepines, taking prazepam can lead to benzodiazepine dependence .
Contraindications and restrictions on use
Prazepam should not be used in children and adolescents under 18 years of age, alcohol or drug addicts. Prazepam should be used with caution in the elderly or in patients with comorbid mental illness . Its use is not recommended during pregnancy and should only be used in exceptional cases when absolutely necessary.
Interactions
Prazepam interacts with some drugs, most notably cytochrome P450 3A4 inhibitors such as B. ritonavir , indinavir , nelfinavir , saquinavir , paroxetine , fluoxetine , fluvoxamine , cobicistat or valproic acid . Together with muscle relaxants, the inhibiting neurons are strengthened in the CNS. This leads to a decrease in muscle tone , which increases the risk of falling. This is especially important for older patients. Opioids or hypertensives (e.g. morphine , piritramide or also clonidine ), together with prazepam, lead to a reduction in the respiratory drive, which in high doses or in pre-stressed people can ultimately lead to respiratory insufficiency. Other drugs with dangerous interactions are trazodone , risperidone, and levomepromazine .
As a precaution, concomitant use of prazepam with antiretroviral therapies should be avoided, as interactions of some benzodiazepines with HIV protease inhibitors (e.g. ritonavir , saquinavir) have been observed.
toxicity
In animal studies, prazepam shows delayed growth and damage in development in embryos.
Trade names
Trade names for Prazepam are Demetrin (CH, D, MZ, PT, SA), Centrac, Centrax, Lysanxia, Mono Demetrin, Pozapam, Prasepine, Prazene, Reapam and Trepidan (USA), Lysanxia (B, F), Centrac (GR, IR), Prazene (IT), Trepidan (IT), Reapam (NL), Pozapam (TH), Prasepine (TH).
Individual evidence
- ↑ Prazepam - Safety data sheet according to 1907/2006 / EG / Article 31. (PDF; 53.3 KB) Version number 4. In: fagron.com. July 17, 2014, accessed August 10, 2018 .
- ↑ a b c d e Prazepam data sheet at Sigma-Aldrich , accessed on February 27, 2017 ( PDF ).
- ^ A b Richard Lawrence Miller: The Encyclopedia of Addictive Drugs. Greenwood Publishing Group, 2002, ISBN 978-0-313-31807-8 , p. 388.
- ↑ Science-Online-Lexika: Entry on Benzodiazepine in the Lexikon der Neurologie. Retrieved May 28, 2009.
- ↑ a b c d Franz v. Bruchhausen: Hager's handbook of pharmaceutical practice. Springer-Verlag, 2013, ISBN 978-3-642-57880-9 , p. 311.
- ↑ a b c d Specialist information Demetrin / Mono Demetrin ( Pfizer Pharma PFE GmbH ), as of June 2016.
- ↑ Danion JM, Brion S, Escande M, etal: Treatment of anxiety with prazepam, 40 mg. A controlled study versus lorazepam . In: Encephale . 10, No. 3, 1984, pp. 135-138. PMID 6389091 .
- ↑ Dièye AM, Sy B, Diarra M, Faye B: Prescription and use of benzodiazepins in Saint-Louis in Senegal: patient survey . In: Ann Pharm Fri . 62, No. 2, March 2004, pp. 133-137. PMID 15107731 .
- ↑ Shader RI, Pary RJ, Harmatz JS, Allison S, Locniskar A, Greenblatt DJ: Plasma concentrations and clinical effects after single oral doses of prazepam, clorazepate, and diazepam . In: J Clin Psychiatry . 45, No. 10, October 1984, pp. 411-3. PMID 6148339 .
- ↑ Ansseau M, von Frenckell R, Jacqmin P: Comparison of sublingual and oral prazepam in normal subjects. I. Clinical data . In: Neuropsychobiology . 18, No. 2, 1987, pp. 77-82. doi : 10.1159 / 000118397 . PMID 3330182 .
- ↑ JP Chabannes, P. Lemoine: [Prazepam drops versus 10 mg prazepam tablets in anxious patients in ambulatory care]. In: Therapy. Volume 45, Number 6, 1990 Nov-Dec, pp. 467-470, PMID 2080484 .
- ↑ N. Authier, D. Balayssac, M. Sautereau, A. Zangarelli, P. Courty, AA. Somogyi, B. Vennat, PM. Llorca, A. Eschalier: Benzodiazepine dependence: focus on withdrawal syndrome. . In: Ann Pharm Fri . 67, No. 6, November 2009, pp. 408-13. doi : 10.1016 / j.pharma.2009.07.001 . PMID 19900604 .
- ↑ a b c d Flockhart DA: Drug Interactions: Cytochrome P 450 Drug Interaction Table . Indiana University School of Medicine. 2007. Retrieved on December 25, 2008.
- ↑ Fox KA, Guerriero FJ: Effect of benzodiazepines on age of vaginal perforation and first estrus in mice . In: Res. Commun. Chem. Pathol. Pharmacol. . 21, No. 1, July 1978, pp. 181-184. PMID 28555 .
- ↑ Guerriero FJ, Fox KA: Benzodiazepines and reproduction of swiss-webster mice . In: Res. Commun. Chem. Pathol. Pharmacol. . 13, No. 4, April 1976, pp. 601-10. PMID 4863 .
- ↑ Guerriero FJ, Fox KA: Benzodiazepine-induced suppression of estrous cycles in C57BL / 6J mice . In: Res. Commun. Chem. Pathol. Pharmacol. . 11, No. 1, May 1975, pp. 155-158. PMID 239442 .
- ↑ Benzodiazepine Names . non-benzodiazepines.org.uk. Archived from the original on December 8, 2008. Info: The archive link was automatically inserted and not yet checked. Please check the original and archive link according to the instructions and then remove this notice. Retrieved May 31, 2009.