Eastwood olefination
The Eastwood olefination or Eastwood deoxygenation is a reaction in the field of organic chemistry . It was discovered in 1964 by G. Grank and FW Eastwood and named after Eastwood. It deals with the conversion of a diol into an alkene (olefin).
Overview reaction
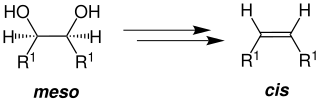
This reaction describes the stereospecific preparation of alkenes from vicinal diols with the aid of a catalytic amount of acid (e.g. acetic acid ).
After several reaction steps, an olefin is formed from a vicinal diol (R 1 = H, alkyl group ).
mechanism
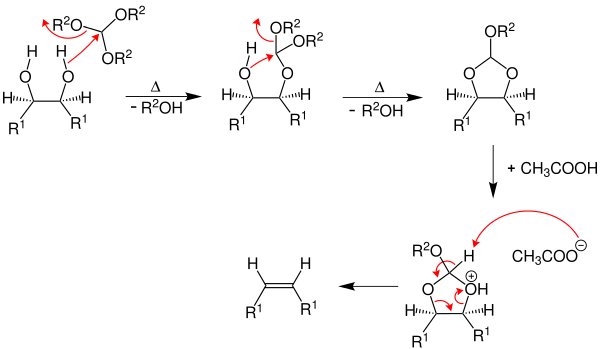
By using acetic anhydride as a solvent, a high yield of alkenes can be achieved.
First, the vicinal diol is reacted with triethyl orthoformate. This is followed by thermal decomposition , with a primary alcohol being split off. This reaction step is repeated. An intermediate product is formed which is converted into an alkene with the aid of a carboxylic acid (here for example acetic acid ) (R 1 = H, alkyl group, R 2 = ethyl group ).
Individual evidence
- ^ G. Crank, FW Eastwood: Derivatives of orthoacids. I. Bicyclic orthoesters. In: Australian Journal of Chemistry. 17, No. 12, 1964, pp. 1385-1391, doi: 10.1071 / CH9641385 .
- ↑ a b c Z. Wang (Ed.): Comprehensive Organic Name Reactions and Reagents. 3 Volume Set, John Wiley & Sons, Hoboken, NJ, 2009, ISBN 978-0-471-70450-8 , p. 949.
- ↑ Andrew G. Myers (Harvard University): Advanced Organic Chemistry - Synthesis of Complex Molecules : Reduction ( Memento of October 19, 2013 in the Internet Archive ) (Handout), accessed December 10, 2013.